- Home
- ComplicAID Home
- Basics Book


TABLE OF CONTENTS
INTERVENTION DEFINITIONS
Successful Procedure
- Angiographic Success: < 30% diameter obstruction with ≥ TIMI II flow post-procedure
- Procedural lesion Success: < 30% diameter obstruction in with ≥ TIMI III flow post-procedure of at least 1 lesion
- Clinical Success: angiographic success of at least one lesion and no major complications
Procedural Complications
- Transient abrupt closure: reversible complete closure of the treated vessel at anytime during PCI
- Persistent closure: complete occlusion of the treated vessel with <TIMI II flow at the end of PCI
- Acute closure: post-procedure out of the cath lab occlusion of the treated vessel
- Stent thrombosis: acute/sub-acute closure in the stented vessel within 4 weeks post stenting
- Slow flow: delayed distal clearance of the dye in the absence of proximal dissection/ spasm
- No flow: no clearance of the dye in the absence of proximal dissection/spasm
- Distal thromboembolism: visible translucent filling defect or abrupt cutoff in the distal vessel
- Air embolism: air injection into coronary tree: non-significant or significant requiring treatment
- Coronary spasm: focal/diffuse narrowing of vessel without any evident coronary dissection
- Side-branch closure: 1.5 mm diameter with normal flow pre-procedure (side-branch intervention due to complication is called side-branch closure requiring intervention; planned bifurcation intervention should not be called side-branch intervention)
- Coronary dissection: intimal flaps NHLBI grade A, B, C, D, E, F
- Coronary perforation: dye outside the vessel lumen: blush, extravasation or free spilling in pericardium
- Prolonged hypotension: systolic blood pressure 5 min
- Persistent chest pain: chest pain lasting for > 30 min post-procedure
- Contrast Induced Nephropathy (CIN): absolute rise of 0.5mg% of S.Cr above the baseline
Vascular Complications
- Hematoma: small 3-5 cm, moderate = 5-10 cm, large > 10 cm
- Pseudoaneurysm requiring prolonged compression / surgical repair
- Bleeding complication (TIMI)
- Major: CVA, ↓ Hb > 5 g%, coronary perforation, or fatal bleeding.
- Minor: ↓ Hb 3 – 5 g% without any other obvious bleeding source or > 3 g% with other obvious bleeding source
- Insignificant: ↓ Hb < 3 g%
- Bleeding requiring transfusion
- Vascular surgery in-hospital or requiring readmission
- A-V fistula
- Thromboembolism
DEFINITIONS AND THRESHOLDS
Cardiac Catheterization Complication thresholds
- Major complications: Q-wave or large non-Q-wave MI (CK-MB > 10x), stroke, emergency CABG, or procedure related death; Threshold < 0.32%
- Minor complications: Non-Q-wave MI (CK-MB 3-10x), acute closure, renal failure requiring dialysis, allergic reaction, cardiac tamponade, arrhythmia; Threshold < 0.5%
- Vascular complications: Hematoma > 5 cm, pseudo aneurysm requiring extra treatment, vascular surgery in-hospital or requiring readmission, A-V fistula, thromboembolism; Threshold < 0.5%
- Procedural mortality: Procedure-related death within 24 hours of cath or later due to complication of cardiac cath; Threshold <0.17%
Coronary Intervention Complications Thresholds
- Major complications: Q-wave MI, large non-Q-wave MI with CK-MB > 10x normal, acute closure or stent thrombosis with CK-MB elevation > 10x normal, emergency CABG, procedure related death, or stroke; Threshold < 1%
- Minor complications: Procedural complication, acute closure or stent thrombosis with CK-MB elevation > 3x normal, a asymptomatic 5-10x normal, TIA, coronary perforation requiring treatment, contrast anaphylaxis, vascular surgery, renal failure with dialysis; Threshold < 3%
- Vascular complications: Hematoma > 5cm, pseudoaneurysm requiring extra treatment, vascular surgery in-hospital or requiring readmission, A-V fistula, thromboembolism; Threshold < 1.5%
- Procedural mortality: Procedure-related death within 24 hrs of intervention or later due to complication of intervention; Threshold < 0.3%
- In-hospital mortality: In-hospital non-risk adjusted death later due to any cause (procedure related or unrelated); Threshold < 1.0%
TIMI Flow
Thrombus Grades
References
- Sharma S., Dangas G., et al. Risk Factors for the Development of Slow Flow During Rotational Coronary Atherectomy. Am J Cardiol (1997) 80:219–222
- Kini A., Marmur J., et al. Creatine Kinase-MB Elevation After Coronary Intervention Correlates With Diffuse Atherosclerosis, and Low-to-Medium Level Elevation Has a Benign Clinical Course. JACC (1999) 34:663–671
- Ryan T., Faxon D., et al. Guidelines for Percutaneous Transluminal Coronary Angioplasty. Circulation (1988) 78:486–502
CORONARY PERFORATION
- Coronary perforation although rare is one of the most feared complication of percutaneous coronary intervention (PCI)1
- Incidence: 0.4%2
- Risk factors:3
- Chronic total occlusions
- Angulated calcified type B2 and type C lesions
- Long lesions (>10 mm)
- Eccentric lesions
- Smaller vessel size
- Older age
- Female sex
- Renal failure
- Previous coronary artery bypass graft surgery
- Common causes:3
- Oversizing of the dilatation catheter and balloon/stent mismatch [Balloon - artery ratio >1.3/1]
- Inflation of a non-compliant balloon to very high pressures
- Use of atheroablatives devices or cutting balloons
- IVUS directed optimal PCI with high pressure stenting
- Classification of coronary perforation: There are two classification schemes for coronary perforation - Ellis4 and Kini classification.5 Ellis classification scheme, more commonly used describes wire and device perforations into following categories:
| Type I | Extraluminal Crater without extravasation |
| Type II | Pericardial or myocardial blush without a ≥1mm exit hole and without contrast jet extravasation |
| Type III | Frank extravasation of contrast and a ≥1mm exit hole |
| Type III- Cavitary Spilling (CS) | Perforation into an anatomic cavity chamber, such as the coronary sinus, or the right ventricle |
Kini classification scheme is more simplistic, focused on wire perforations and describes two types of wire perforations:
- Type I described as "myocardial stain" with no frank dye extravasation and
- Type II as "myocardial fan" with dye extravasation into pericardium, coronary sinus, or cardiac chambers
- A significant proportion of perforations occur with guidewires crossing the lesion, with distal wire perforation or wire fracture. Extra stiff wires and low friction hydrophilic-coated wires are associated with higher incidence of perforation.6,7 This may reflect either use of specialty wires to facilitate passage through more complex lesions or their ease of distal migration.
- Prevention: meticulous attention to guidewire position, careful and appropriate sizing of the balloon or stent prior to inflation, and avoiding over dilation or high pressure inflation exceeding the balloon's burst pressure
- Management: Clinical suspicion should rise if patient develops sudden onset of acute/sharp chest pain or have sudden explained severe hypotension, particularly when inflating balloon or deploying a stent. If clinical suspicion arises, pull balloon immediately into the guide and perform angiography to confirm diagnosis.
- The first aim is to prevent cardiac tamponade by immediate balloon inflation [SDS or the balloon present in the guide] proximal or at site of perforation at the lowest pressure
possible. Usually 2-4 atmospheres for about 5-10 minutes is sufficient. However, may need to go to higher pressure and or longer duration to achieve hemostasis. Assess for hemostasis throughout intervention by injecting contrast at regular intervals.
- Consider anticoagulation reversal: Decision to reverse needs to be balanced against potential risk of acute thrombosis, especially if a stent was just deployed. Heparin reversal: protamine sulfate 1mg IV/100 units of UFH (to achieve activated clotting time of <150s). Bivalirudin reversal: fresh frozen plasma is preferred and it results in partial reversal.
- Aggressive treatment with intravenous fluids, atropine, vasopressors,
mechanical circulatory support may be required if hemodynamics deteriorate. Call CT surgery for backup.
- Emergent bedside echocardiogram should be obtained. If patient has significant effusion with tamponade physiology, perform emergent pericardiocentesis.
Treatment of coronary perforation
Type 1 perforation- Often resolves without intervention and reversal of anticoagulation
- If above measure fails, perform prolonged balloon inflation (10-15 min) proximal or at site of injury
- If still persists, follow steps for type II/III/III CS perforations as explained below
- Prolonged balloon Inflation proximal or over perforation site and reversal of anticoagulation. If still bleeding, repeat prolonged balloon inflation
- If extravasation persists, seal the site with either occlusive coils [perforation site distal main vessel] or by implantation of polytetrafluoroethylene (PFTE) covered stent [perforation site proximal main vessel, distal side branch which can be excluded with covered stent]
- If extravasation still persists or site of injury is proximal main vessel with bifurcation (covered stent not an option) consider emergent surgery
- Type III CS draining into coronary sinus or right ventricle is usually benign and can be managed conservatively
- Reverse anticoagulation.
- Inflate appropriately sized balloon to low atmospheric pressure proximal or at the site of perforation and confirm sealing of further extravasation with contrast injection from guiding catheter.
- Perform prolonged balloon inflation (10–20 min) proximal or at the site of perforation if the perforation is in distal territory or in tertiary branches.
- Deflate balloon and perform contrast injection- if persistent extravasation, reinflate balloon and start preparing for coil delivery microcatheter placement.
- Remove the balloon and track the coil delivery microcatheter over the guide wire and place it about 1 mm proximal to the site of perforation.
- Load the occlusive coil into the microcatheter and advance it by pushing with either a 0.018” guidewire or the stiffer backend of workhorse guidewire. Push the coil out distally and withdraw the catheter simultaneously.
- Consider delivery of second coil if there is persistent leak.
- In some cases with persistent coronary leak from a side branch, a covered stent can be placed in the main vessel, cutting off the blood supply to the side branch with resultant resolution or minimization of leakage.
- Perform transthoracic echocardiogram on procedure table to rule out large pericardial effusion and perform emergent pericardiocentesis if evidence of tamponade.
- Monitor in the coronary care unit and obtain an echocardiogram the following day.
- Withhold antiplatelet agents for 12–24 hours and resume usual dose once uneventful.
References
- Shimony A, Joseph L, Mottillo S, Eisenberg MJ. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol 2011;27:843–50.
- Kinnaird T, Kwok CS, Kontopantelis E, et al. Incidence, determinants and outcomes of coronary perforation during percutaneous coronary intervention in the United Kingdom between 2006 and 2013. An analysis of 527121 cases from the British Cardiovascular Intervention Society Database. Circ Cardiovasc Interv 2016;9:e003449.
- Ellis SG, Roubin GS, Kinh SB, et al. Angiographic and clinical predictors of acute closure after native vessel coronary angioplasty. Circulation 1988;77:372–9.
- Ellis S.G., Ajluni S., Arnold A.Z., et al. (1994) Increased coronary perforation in the new device era. Incidence, classification, management, and outcome. Circulation 90:2725–2730.
- Kini AS, Rafael OC, Sarkar K, et al. Changing outcomes and treatment strategies for wire induced coronary perforations in the era of bivalirudin use. Catheter Cardiovasc Interv. 2009;74(5):700‐707. doi:10.1002/ccd.22112.
- Al-Lamee R., Ielasi A., Latib A., et al. (2011) Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforations. J Am Coll Cardiol 4:87–95.
- De Marco F., Balcells J., Lefèvre T., Routledge H., Louvard Y., Morice M.C. (2008) Delayed and recurrent cardiac tamponade following distal coronary perforation of hydrophilic guidewires during coronary intervention. J Invasive Cardiol 20:E150–E153.
ABRUPT VESSEL CLOSURE (AVC)
- AVC is the commonest major complication of PCI1
- Incidence: 0.3% [used to be 3% in pre-stent era]2
- Risk factors:3
- Proximal vessel tortuosity
- Diffuse lesion
- Pre-existing thrombus
- Degenerated vein graft
- Extremely angulated lesion
- Unstable angina
- Multivessel disease
- Female gender
- Chronic renal failure
- Common causes:3
- Coronary dissection
- Intracoronary thrombus formation
- Native thrombus (or atheroma) embolization
- Air injection
- Coronary no-reflow
- Coronary vasospasm
- Classification of coronary perforation: As per the National Heart, Lung and Blood Institute scheme, types A–F classification remains useful to describe the severity of luminal injury:4
| Type A | Minor radiolucency within the coronary lumen without dye persistence |
| Type B | Parallel tracks or double lumen separated by a radiolucent area during angiography without dye persistence |
| Type C | Extraluminal, persisting extravasation of contrast |
| Type D | Spiral luminal filling defects |
| Type E | Persistent lumen defect with delayed antegrade flow |
| Type F | Filling defect accompanied by total coronary occlusion |
- Prevention:
- Maintain ACT > 300 throughout procedure
- Make sure interface is free of air
- Avoid high-pressure balloon dilatation or stenting
- Avoid unnecessary post-dilatation and very long stents
- Use distal protection devices in vein graft PCI
- Be careful when retrieving delivery after stent implantation
- Avoid geographical miss during stenting
- Avoid aggressive post-dilatation at the stent edges
- Be careful while positioning wire distal tip in tortuous vessel
- Management: Abrupt closure results in acute ischemia manifesting as ECG changes, hypotension, bradycardia, chest pain and ventricular arrhythmias. The first step is to identify the underlying cause of AVC and then treat it accordingly.
- Immediate priority should be to ensure intraluminal position of coronary guidewire and, if in doubt, an over-the-wire balloon catheter or Twin-Pass or other microcatheter should be advanced distal into the target vessel to allow minimal contrast media injection and confirm wire position.
- If intraluminal guidewire position is confirmed, the most likely mechanism underlying AVC is dissection or intraluminal thrombus. Prompt balloon
inflation should be attempted to establish antegrade flow. If flow returns immediately after balloon inflation the likely cause of AVC is dissection and urgent stenting is useful for stabilizing.
- If the distal flow after balloon inflation is sluggish (TIMI 0 or 1), the likely cause of AVC is distal thromboembolism. Using a Twin-Pass or microcatheter to administer distal vasodilators can help reestablish flow.
- If initial contrast agent injection reveals guidewire position within a false lumen, careful exploration of the occluded segment using a second guidewire must be performed.
- Aspiration thrombectomy and Glycoprotein IIb/IIIa antagonists may be helpful if acute closure is due to
thrombus. Control of anticoagulation is of paramount importance to avoid thrombotic occlusion of stented artery. ACT should be measured every ~30 minutes to keep ACT > 300 throughout the procedure and dose of anticoagulation is adjusted accordingly. If ACT is not reaching therapeutic levels consider resistance to anticoagulant and a possible reason for suspected thrombus formation causing AVC.
- Intravenous fluids, vasopressors, inotropes and intra-aortic balloon pump (IABP) should be considered for unstable hemodynamics.
- Emergency CABG should be considered if patient have persistent AVC depending on the location of the occlusion, patient’s clinical condition and assessment of risks and benefits.
Coronary slow flow/no-reflow phenomenon
Slow flow/No-reflow is an acute reduction in coronary flow (TIMI grade 0–1) in a patent vessel with absence of dissection, thrombus, spasm, or high-grade residual stenosis at the original target lesion.5 The underlying mechanism is complex and not completely understood, but some proposed mechanisms include distal embolization of calcium, plaque or thrombus and microvascular spasm caused by release of vasoconstrictor substances like serotonin and thromboxane, oxidative stress, and reperfusion injury.6 Clinical and lesion characteristics associated with higher incidence of no-reflow include left ventricular systolic serotonin and thromboxane, oxidative stress,- Direct stenting whenever feasible
- Use of distal embolic protection devices for vein graft interventions.
- Aspiration thrombectomy in STEMI cases if there is large thrombus burden
- For cases involving rotational atherectomy, the use of rota flush, small
initial burr sizes, shorter rotablation runs, avoiding drops in rotations per minute (RPMs), and prevention of hypotension/bradycardia
Management: Coronary no re-flow must be immediately differentiated from AVC due to dissection as placement of stent in a vessel with no reflow may worsen the situation. Exclusion of dissection, thrombus, spasm, or high-grade residual stenosis at the original target lesion suggests no-reflow.- Stabilize hemodynamics with medications/intra-aortic balloon pump (IABP)
- IC verapamil (100–200 μg)
- IV adenosine (10–20 μg)
- IC nitroprusside (50–200 μg)
- Moderately forceful injection of blood or saline through the manifold
- GPIIb/IIIa agents, IV cangrelor may also be helpful
Air Embolism
Intracoronary air embolism is a potentially lethal but rare complication. It could result in hypotension, hemodynamic collapse, cardiac arrest, and in rare cases death. Coronary air embolism is almost always iatrogenic. It occurs mostly when- Catheters are not adequately aspirated and flushed
- During introduction or withdrawal of a guidewire, balloon catheter or other interventional devices
- Rupture of a balloon during high inflation
- During intracoronary medication injection
- Do not engage the left main coronary when pulling out the guiding wire unless the patient has excessive aortic tortuosity or an enlarged aortic root.
- Do not connect the manifold to the catheter with the flush running. This may lead to an air embolism if the catheter already has a column of air inside it.
- Draw back at least 2 cc of blood into the injection syringe and make sure that the interface is free of air prior to injection.
- Inject some dye into the ascending aorta prior to engaging left main.
- Always ensure that all the catheters and tubings are aspirated, flushed and free of air.
- Take adequate care when prepping stents or balloons and ensure that the syringe tip is facing downwards.
- Always inject with the syringe tip facing downwards
- Put patient on 100% oxygen.
- Flush air free saline vigorously into the coronary arteries. Aspirate blood and air column via guide catheter and reinject saline forcefully back into coronary arteries.
- Administer IV phenylephrine 200 μg for hypotension. Repeat, as needed every minute. If significant hypotension or hemodynamic collapse is present, push IV 1 cc epinephrine (1:10,000 dilution).
- Intracoronary injection of vasodilators (adenosine, nitroprusside, verapamil) may be attempted.
- Supportive measures should be instituted (IABP for persistent hypotention) and patient admitted to intensive coronary care unit for further monitoring
Coronary Vasospasm
Coronary vasospasm can be induced by PCI secondary to endothelial denudation and nitric oxide loss.- Some cases are catheter-induced which is caused by a contact of a catheter without balloon deployment. It is usually short-lived and is most prone to occur at the ostium of the right coronary artery (RCA). The left main is less susceptible to ostial spasm
- Rotablator cases are more prone to vasospasm
- Coronary vasospasm is detected by presence of EKG changes of ST segment elevation in association with angina, and then EKG completely returns to baseline upon resolution of symptoms.
- The definitive diagnosis of coronary vasospasm is made angiographically by demonstration of reduction of luminal diameter in a discrete segment of the vessel, which is proven reversible by the administration of intracoronary vasodilators.
- Initial step is intracoronary vasodilatation with IC calcium channel blockers and/or nitrates [nitroglycerin 100-300 mcg, verapamil 100 mcg/min, up to 1.0-1.5 mg, nicardipine 100-300 mcg, nitroprusside 100-300 mcg]
- IV atropine can be useful if there is associated hypotension of bradycardia
- If vasospasm persists, remove all hardware and leave the guide wire in place to maintain position. This may
If vasospasm persists, remove all hardware and leave the guide wire in place to maintain position. This may minimize distal vessel spasm
- Repeat prolonged PTCA for 2-5 minutes at low pressures (1-4 atmospheres)
- Stenting should be reserved in cases if all the above measures have failed, as it may lead to propagation of spasm to a new location
- Refractory vasospasm may be indicative of dissection, which is also an indication for stenting
Abrupt Vessel Closure Summary
- Dissection
- Minor dissections - usually heal without clinical sequelae, no treatment required
- Major dissections - repeated prolonged low-pressure balloon [distal vessel], stenting [Proximal/mid vessel segment or impaired flow due to dissection]
- Thromboembolism
- Twin-Pass or microcatheter to administer vasodilators distally
- Check ACT to keep > 300. Consider starting IV Cangrelor or bailout GPIs
- Balloon dilatation and/or thrombus aspiration in case of stent thrombosis
- Stenting on case of thrombosis in in unstented vessel segment
- No-reflow
- Intracoronary Adenosine, Nitroprusside, Nicardipine, Verapamil, or GPI’s
- A transit catheter or over-the-wire balloon should be used to deliver the vasodilators to the distal microvasculature
- Insertion of IABP to improve flow
- Air embolism
- Start 100% oxygen
- Flush air free saline vigorously into the coronary arteries. Aspirate blood via guide catheter and reinject forcefully back into coronaries
- IV phenyl epinephrine or epinephrine as needed
- Intracoronary injection of vasodilators
- Vasospasm
- Intracoronary Nitroglycerin, Adenosine, Nitroprusside, Nicardipine, or Verapamil
- IV fluid bolus and/or atropine as needed
- Remove all hardware and leave the guide wire in place to maintain position
- Repeat prolonged PTCA for 2-5 minutes at low pressures (1-4 atmospheres)
- Unknown etiology
- Maintain wire position distally and pass a microcatheter distally to inject contrast
- If flow distally, problem at site of vessel closure and needs to be investigated
- If no flow distally, consider no reflow and give IC vasodilators
References
- de Feyter P.J., de Jaegere P.P.T., Murphy E.S., Serruys P.W. (1992) Abrupt coronary artery occlusion during percutaneous transluminal coronary angioplasty. Am Heart J 123:1633–1642.
- Francesco Giannini, Luciano Candilio, Satoru Mitomo, Neil Ruparelia, Alaide Chieffo, Luca Baldetti, Francesco Ponticelli, Azeem Latib, Antonio Colombo. Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. J Am Coll Cardiol Intv. 2018 Sep, 11 (18) 1797-1810.
- Klein L. (2005) Coronary complications of percutaneous coronary interventions: a practical approach to the management of abrupt closure. Catheter Cardiovasc Interv 64:395–401.
- Huber MS, Mooney LF, Madison J, et al. Use of a morphologic classification to predict clinical outcome after dissection from coronary angioplasty. Am J Cardiol 1991;68:467–71.
- Rezkalla S.H., Kloner R.A. (2002) No-reflow phenomenon. Circulation 105:656–662.
- Piana R., Paik G., Moscucci M., et al. (1994) Incidence and treatment of “no-reflow” after percutaneous coronary intervention. Circulation 89:2514–8.
ROTATIONAL ATHERECTOMY COMPLICATIONS
- Common complications associated with rotational atherectomy are:1
- Slow flow / No-reflow
- Coronary Dissection
- Coronary Perforation
- Burr entrapment
Slow flow / No-reflow
- It is most feared and preventable operator-dependent complication of Rotational Atherectomy (RA)
- Incidence- 2.6% in the drug-eluting stent era2
- Omens of slow-flow / no-reflow include sudden decelerations and visual, tactile or auditory clues of high resistance to burr advancement
- Be mindful of incident chest pain, ST-segment elevations, hemodynamic instability, and bradyarrhythmia while burring which could signal no-reflow phenomenon
- Prevention
- Optimal antiplatelet and anticoagulant therapy
- Continuous flush cocktail
- Smaller burr sizes (Max burr to artery ratio 0.4-0.6)
- Lower speeds (140-150K rpm)
- Short ablation runs of 15-20 seconds
- Pause between runs
- Treatment
- Correction of hypotension with fluids, vasopressors, and pacing as required
- Administration of intracoronary vasodilators, such as adenosine, nitrates, nitroprusside, nicardipine, and verapamil administered distally in the vessel
- If hemodynamically unstable, insertion of an intraaortic balloon pump to augment coronary perfusion pressure
Coronary Dissection
- Dissections during RA are described and graded in standard fashion using the NHLBI classification system (A-F)
- Incidence: 1.7%- 5.9% in the drug-eluting stent era 3
- Like slow-flow / no-reflow, dissection can present with signs and symptoms of acute myocardial ischemia including chest pain, ST-segment elevations, and hemodynamic or electrical instability
- Prevention:
- Avoid rotablation in excessively tortuous vessels
- Avoid excessive angulation while burring
- Smaller burr sizes
- Treatment:
- Stop further ablation
- Maintain wire position
- Expeditious completion of PCI via balloon angioplasty and stenting if feasible
Coronary Perforation
- Perforation represents a more severe variant of dissection in which disruption extends through the full thickness of the arterial wall.
- Incidence: 0-2% in the drug-eluting stent era3
- Coronary perforations during RA are described and graded in standard fashion using the Elis classification scheme (I-III)
- Although RA is considered a risk factor for perforation,4 the majority of type III perforations result from balloon angioplasty 5
Coronary Perforation
- Risk factors: lesion-specific predictors of perforation include eccentricity, tortuosity, length >10 mm, and location in the right coronary artery or left circumflex artery
- Prevention:
- Correct burr sizing
- Avoid aggressive burring
- Avoid excessive angulation
- Lower speeds
- Treatment:
- Stop further ablation
- Maintain wire position
- Discontinuation of anticoagulation
- Prolonged balloon inflation (10-15 min) proximal or at site of injury. If still bleeding, repeat prolonged balloon inflation
- If extravasation persists, seal the site with either occlusive coils [perforation site distal main vessel] or by implantation of polytetrafluoroethylene (PFTE) covered stent [perforation siteproximal main vessel, distal side branch which can be excluded with covered stent]
- If extravasation still persists or site of injury is proximal main vessel with bifurcation (covered stent not an option) consider emergent surgery
- Aggressive treatment with intravenous fluids, atropine, vasopressors, mechanical circulatory support if hemodynamics deteriorate
Burr Entrapment
- Entrapment consists of burr embedding in a severe stenosis, preventing both further burr advancement and retrieval
- Presence of diamond chips on the front, but not the rear, of the burr abets an opportunity for the burr to lodge within a lesion and become entrapped.
- Once stuck and stalled within a lesion, retrograde ablation is not possible and friction associated with retrograde motion cannot be orthogonally displaced.
- During ablation, the operator should be attentive to potential warning signs, which may be visual (lack of smooth advancement under fluoroscopy), auditory (pitch changes with variation in resistance encountered by burr), or tactile (resistance in advancer knob or excessive driveshaft vibration)
- Incidence: 0.5% to 1%3
- Prevention:
- Meticulous relief of system tension before RA
- Gentle pecking motions
- Short ablation runs
- Avoid excessive tortuosity
- Do not stop spinning within a lesion
- Treatment:
- Apply forceful pull on the Rota wire with guide disengaged taking advantage of the wire’s 0.014 inches spring tip
- Administer high dose of vasodilators and aggressively pull the Rota burr
- Manual traction with on-Dynaglide or off-Dynaglide rotation
- If above measures fail, potential catheter-based solutions to facilitate burr retrieval include
- Obtain second arterial access and advance Fielder wire and a small (1~1.25mm) balloon distally, inflate at the level of Rota burr, then aggressively pull the Rota burr
- Advance Guide extension catheters on the Rota Burr
- Cut the Rota burr and aggressively pull the Teflon covering sheath.
- once done, then advance 6Fr Guide extension on the shaft until the Rota burr and pull aggressively
- Subintimal tracking and reentry with balloon dilatation adjacent to the entrapped burr6, 7
ORBITAL ATHERECTOMY COMPLICATIONS
Differential sanding of Orbital atherectomy (OA) permits healthy tissue to flex away from the crown during orbit and can be used with speed selection options for low speed (80,000 rpm), high speed (120,000 rpm), or GlideAssist (5000 rpm).
Common complications associated with rotational atherectomy are:
- Slow flow / No-reflow
- Coronary Dissection
- Coronary Perforation
Slow flow / No-reflow
Incidence: 0.9% in Orbit II trial and 0.7% in real work registry analysis.8, 9The unique mechanism of action, differential sanding, combined with an average particle size of debris of 2.04 μm – smaller than a red blood cell – may contribute to lower rates of no-reflow and transient heart block with orbital atherectomy.10
Prevention- Optimal anticoagulant and antiplatelet therapy
- Continue ViperSlide infusion
- Always keep the crown advancing or retracting with slow advancement (1mm/sec)
- Short run timetime ( <20 seconds)
- Rest time = or > run time
- Ensure to keep the optimal blood pressure (SBP > 100Hg) and give fluids, vasopressors, and pacing as needed
- Administer intracoronary vasodilators, such as adenosine, nitrates, nitroprusside, nicardipine, and verapamil administered distally in the vessel if necessary, via twin-pass dual access catheter
- If hemodynamically unstable, place an intra-aortic balloon pump to augment coronary perfusion pressure
Coronary Dissection
- Coronary artery dissection can be categorized by using NHLBI classification system (A-F)
- Incidence: 3.4 % in Orbit II trial and 0.9% in Real world registry8, 9
- Dissection can manifest with acute onset of chest pain, new EKG changes with ST elevations, and hemodynamic or conduction disturbances.
- Avoid high speed run
- Avoid in very tortuous coronary anatomy or > 2 bends exceeding 90° angulations
- Use of ViperWire advance with flex tip in a setting of tortuous artery
- Stop ablation immediately
- Reassess hemodynamic and patient status, then give vasopressor as needed
- Completion of PCI with balloon angioplasty and stent placement if possible
Coronary Artery Perforation
- Coronary perforation is the most serious complication that can occur with OA.
- With unique mechanism with pulsatile forces in OA, it can result in more significant tissue modification while having a higher risk of deep dissections and perforation.
- Incidence: 0.7-2%8, 9, 11
- Coronary perforations during OA can be graded in standard fashion using the Elis classification scheme (I-III).
- Use of lower speed (80,000 rpm)
- Avoid excessive angulation( >2 bends exceeding 90° angulations)
- Careful advancement when evidence of wire wrinkling from tension buildup is present leading to vessel straightening
- Avoid high speed if the vessel diameter is less than 3 mm
- Advance the burr slowly with a speed of 1 mm per second
- Stop further ablation immediately
- Maintain wire position
- Discontinuation of anticoagulation
- Prolonged balloon inflation (10-15 min) proximal or at site of injury. If still bleeding, repeat prolonged balloon inflation
- If extravasation persists, consider to use coils or covered stent
- Reassess the perforation and patient status with angiogram
- Be ready to do emergency pericardiocentesis if necessary
- If extravasation remains present and/or site of injury is proximal main vessel with bifurcation (covered stent not an option), consider emergent surgery
- Aggressive treatment with intravenous fluids, atropine, vasopressors, mechanical circulatory support if hemodynamics deteriorates
References
- Sharma SK, Tomey MI, Teirstein PS, et al. North American Expert Review of Rotational Atherectomy. Circ Cardiovasc Interv. 2019;12(5):e007448. doi:10.1161/CIRCINTERVENTIONS.118.007448
- Naito R, Sakakura K, Wada H, Funayama H, Sugawara Y, Kubo N, Ako J, Momomura S. Comparison of long-term clinical outcomes between sirolimus-eluting stents and paclitaxel-eluting stents following rotational atherectomy.Int Heart J.2012; 53:149–153
- Tomey MI, Kini AS, Sharma SK. Current status of rotational atherectomy.JACC Cardiovasc Interv.2014; 7:345–353. doi: 10.1016/j.jcin.2013.12.196
- Shimony A, Joseph L, Mottillo S, Eisenberg MJ. Coronary artery perforation during percutaneous coronary intervention: a systematic review and meta-analysis. Can J Cardiol 2011;27:843–50.
- Al-Lamee R., Ielasi A., Latib A., et al. (2011) Incidence, predictors, management, immediate and long-term outcomes following grade III coronary perforations. J Am Coll Cardiol 4:87–95
- Sulimov DS, Abdel-Wahab M, Toelg R, Kassner G, Geist V, Richardt G. Stuck rotablator: the nightmare of rotational atherectomy.EuroIntervention.2013; 9:251–258. doi: 10.4244/EIJV9I2A41
- Tanaka Y, Saito S. Successful retrieval of a firmly stuck rotablator burr by using a modified STAR technique. Catheter Cardiovasc Interv.2016; 87:749–756. doi: 10.1002/ccd.26342
- Chambers JW, Feldman RL, Himmelstein SI, et al. Pivotal trial to evaluate the safety and efficacy of the orbital atherectomy system in treating de novo, severely calcified coronary lesions (ORBIT II). JACC Cardiovasc Interv. 2014;7(5):510-518. doi:10.1016/j.jcin.2014.01.158
- Lee MS, Shlofmitz E, Kaplan B, Alexandru D, Meraj P, Shlofmitz R. Real-World Multicenter Registry of Patients with Severe Coronary Artery Calcification Undergoing Orbital Atherectomy. J Interv Cardiol. 2016;29(4):357-362. doi:10.1111/joic.12310
- Sotomi Y, Shlofmitz RA, Colombo A, et al. Patient selection and procedural considerations for coronary orbital atherectomy system. Interv Cardiol 2016;11:33
- Parikh K., Chandra P., Choksi N., et al: Safety and feasibility of orbital atherectomy for the treatment of calcified coronary lesions: the ORBIT I trial. Catheter Cardiovasc Interv 2013; 81: pp. 1134-1139
- Kini AS, Vengrenyuk Y, Pena J, et al. Optical coherence tomography assessment of the mechanistic effects of rotational and orbital atherectomy in severely calcified coronary lesions. Catheter Cardiovasc Interv. 2015;86(6):1024-1032. doi:10.1002/ccd.26000
LONGITUDINAL STENT DEFORMATION
- Longitudinal stent deformation (LSD) is defined as accidental mechanical distortion or shortening of a stent along its longitudinal axis following stent deployment
- Incidence: 0.2% in contemporary era1
- Risk factors:1
- Calcification
- Ostial disease
- Bifurcation disease
- Use of guide extension catheters
- Balloon post-dilatation
- Greater number of deployed stents
- Most common cause:1 Guide catheter or device (e.g. sharp tipped balloon or IVUS catheter) abutting on proximal stent edge, or poorly deflated or winged balloon or other device catching a mid or distal strut upon withdrawal.
- New-generation (cobalt-chromium or platinum-chromium) stents with thinner struts and less connectors allow successful navigation in complex lesions and make side branch access easier. However, with the reduction of the number of fixed links between cells and the alteration of their geometry partly sacrifices their longitudinal strength, leading to an increased risk LSD.2
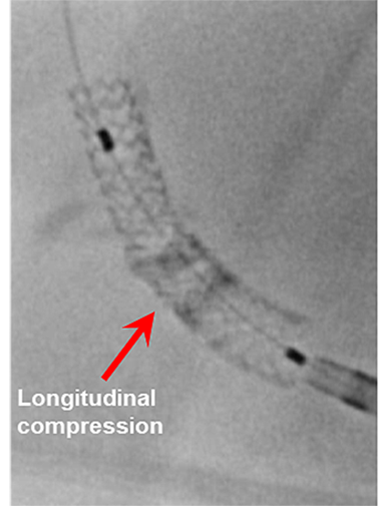
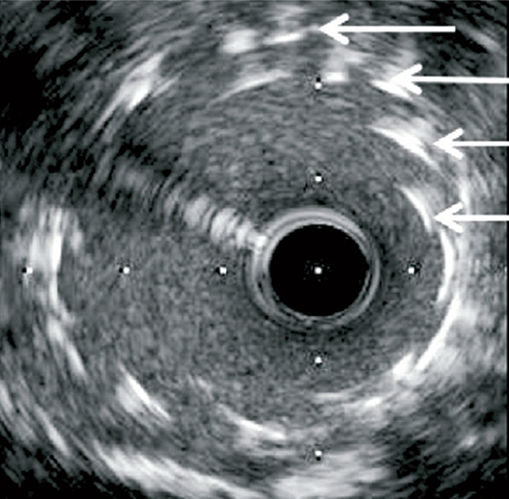
Longitudinal stent deformation: under fluoroscopy (stent compression) and IVUS imaging showing overlapping and distorted struts (concertina pattern)
- LSD can manifest as longitudinal stent compression, which is seen on fluoroscopy as a dark band in the region of compression also called stent accordion, concertina or wrinkling or it can manifest as longitudinal stent elongation which appears like a fracture in the stent (pseudo-fracture).
- The Promus Element stent has been the most frequently reported DES that is vulnerable to LSD, mainly because of its weakness against longitudinal forces, which may be explained by its thin strut and offset peak-to-peak design.2 However, LSD has been reported with other DES, suggesting that risk factors outside of stent platform design may have a role in LSD.2
- LSD while rare, is under-recognized, and may result in:1
- Areas of stent overlap, distortion, malapposition and arterial injury
- Target lesion injury with lack of stent coverage
- Increased risk of stent thrombosis, restenosis and emergent CABG
- Prevention:
- Operator must pay attention during PCI of ostial lesions involving deep intubation with guiding catheters or extension systems through already stented segment
- If resistance to passage of a secondary device in the stent do not push hard
- Use caution following deliberate under expansion of the proximal portion of a very long stent in a tapered vessel as further delivery of additional devices can lead to LSD
- Treatment:
- When LSD is suspected, radiographic assessment of the stented segment, preferably with StentBoost (Philips, Andover, Massachusetts) or an equivalent image-enhancement program, should be done
- Confirm wire position and use a small compliant balloon followed by a high-pressure noncompliant balloon aiming to ensure adequate expansion of deformed stent struts and their apposition to coronary arterial
- If LSD still persists, another stent can be used
- IVUS or optical coherence tomography use is strongly encouraged, although it is advisable to proceed to intracoronary imaging once LSD has been treated to avoid further potential deformation
References
- Kereiakes D.J., Popma J.J., Cannon L.A., et al. (2012) Longitudinal stent deformation: Quantitative coronary angiographic analysis from the PERSEUS and PLATINUM randomised controlled clinical trials. EuroIntervention 8:187–195.
- Ormiston J.A., Webber B., Webster M.W.I. (2011) Stent longitudinal integrity - bench insights into a clinical problem. J Am Coll Cardiol Intv 4:1310–1317.
DEVICE EMBOLIZATION
- Device embolization is a rare PCI complication defined as a loss of a device such as stent, guidewire, catheter fragments or misplaced intravascular coils within the coronary vasculature
- Incidence: Stents are the most common devices embolized, with an incidence ranging from 3% for the first-generation hand-crimped devices to much lower, 0.32%, for current stent delivery system1
- Risk factors:2
- Extreme tortuosity
- Angulation
- Calcified vessels
- Inadequate guide catheter support
- Older generation stents
- Device dislodgement may result in systemic or intracoronary embolization. While systemic embolization may cause severe cerebro-
vascular events, limb ischemia, death and the need for an emergency surgery, intracoronary embolization is associated with high risk of coronary thrombosis and subsequent MI3
- A tortuous and calcified coronary anatomy increases the rigidity of the vessel making it prone to a stent peeling off the balloon as increased force is applied to advance the device. For this reason, stents are more commonly lost in the right coronary and left circumflex arteries and less commonly in the left anterior descending artery4
- Prevention: When facing difficulty delivering a stent:
- Stop and gently retract stent back into the guide catheter and remove it instead of forcing it forward
- Perform pre-dilatation of the lesion if a direct stenting was attempted or if lesion
was predilated perform further dilation using higher atmospheric pressure or bigger balloon or use atheroablative devices for adequate lesion preparation
- Make sure the tip of guide is coaxial especially if there is significant proximal tortuosity
- Use of guide extension catheters can help deliver the stents
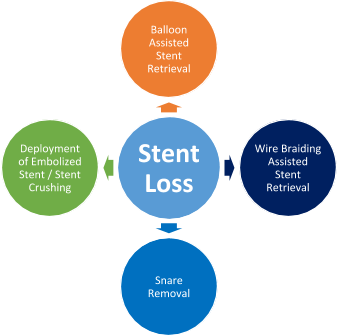
- Management - These are the key steps for managing device embolization during PCI:
- If embolized item is a small guidewire, consider leaving wire in the coronary artery
- No treatment is required for peripherally embolized small stents
- Consider surgical removal if involves larger coronary arteries or difficult vascular access sites or if all the techniques fail to retrieve the device
- Balloon assisted stent retrieval: After withdrawal of the stent balloon from which the stent has become dislodged, a second smaller 1.5 or 2 mm balloon is advanced over the wire and through the stent, and is inflated distal to the stent. Then it is retracted back into the guide. In some cases, the stent may be contained within the distal tip of the guide, but the inflated balloon cannot be retracted into the guide. In this scenario, the guide and balloon should be removed as one unit over the wire. Two balloons are required if the stent diameter is too large for a single balloon to retract.
- Snare removal - This is the most widely used tool for stent retrieval. Many snares are available commercially; gooseneck snare is most commonly used. Once a stent slips off the delivery balloon, the
indwelling wire is advanced as far as possible into the distal vasculature. The loop of the snare is passed over the angioplasty wire, encircles it, and is advanced up to the coronary ostium. It arrives at the right position as its loop is encircling the proximal end of the stent. The loop is tightened by advancing the transport catheter, and the whole stent–snare–wire complex is withdrawn as a unit.
- Wire braiding assisted stent retrieval: - This method is useful if the stent has come off the balloon but remains on the wire. A second, soft-tipped wire is navigated strategically through the side struts and not the central lumen. It is then passed into a branch distal to the stent and is separate from the first wire.
Torque is then applied to both wires, and the twisting action results in them wrapping around both sides of the stent, trapping the stent in between. With gentle and persistent pulling, the whole system (guide, stent twisted between two wires) can be withdrawn. An extension catheter can be used to help retrieve the stent.
- Deployment of embolized stent/Stent crushing or stent exclusion: If attempts for retrieval are unsuccessful, it may be necessary to consider stent crushing to the arterial wall with balloon inflation or deployment of an additional stent alongside the embolized one, although this is associated with an elevated risk of periprocedural MI, death, and referral for CABG. Even if the stent is not advanced
completely through the lesion, it should be expanded where it is to its fullest possible dimension using the deployment balloon. If the deployment balloon cannot be advanced through the stent, a balloon with a lower profile should be inserted.
OSTIAL PCI COMPLICATION
- Percutaneous intervention of ostial lesions in coronary arteries or aortocoronary bypass grafts can pose unique challenges and is associated with higher procedural and medium-term complication rates.5
- Ostial lesion is defined as one arising within 3 mm of the vessel origin. It may be categorized by location as- Aorto-ostial, non aorto-ostial and branch ostial.6
- In the balloon angioplasty era, procedural complications such as dissections, acute vessel closure and myocardial infarction were more frequent.7 This led to interest in the use of adjunctive devices such as directional atherectomy, rotational atherectomy, excimer laser and cutting balloons for the purposes of debulking or modifying plaque at ostial sites, with varying degrees of success. With the
introduction of bare metal stents and drug eluting stents, the restenosis rates and complication rates reduced as compared to balloon angioplasty but the outcomes of placement of DES at ostium still remain inferior to non-ostial lesions.8, 9
- Iatrogenic aortocoronary dissection complicating coronary interventions is extremely rare and a few cases have been reported. The incidence of this complication is approximately 0.02% for diagnostic coronary angiography and 0.02-0.83% for PCI procedures.10
- The rapid propagation of aortocoronary dissection may become life threatening and should be recognized immediately. Most reported iatrogenic aortocoronary dissections have been related to procedures in the RCA, especially during PCI for chronic total occlusions.11
- These are some of the unique complications of ostial PCI:
- The calcified ostium may lead to incomplete stent expansion and predispose to restenosis or stent thrombosis
- Direct contact between the guiding catheter and the proximal edge stent struts may lead to longitudinal stent deformation
- Trauma to the ostium from the guide catheter or during balloon inflation may result in dissection of the coronary artery and aortic root
- There are higher chances of stent misplacement and excessive stent protrusion
Prevention and Treatment of Ostial PCI complications
Guide catheter-induced dissection/occlusion of flow- Selection of less aggressive guide catheters
- Cautious catheter manipulation
- Rapid wiring and pre-loading coronary wire before guide engagement
- Once wire down the vessel try and disengage the guide from ostium to avoid ostial occlusion in case of tight ostial lesions
- Anticlockwise rotation to disengage catheter from RCA ostium
- Aorto-coronary dissection can be successfully managed by stenting of the entry point of the coronary dissection if the dissection extends 40 mm from the ostium or in cases of occlusion of the dissected vessel with cessation of antegrade flow that cannot
be restored percutaneously, and if the extension of the dissection is up to the descending aorta12
Misplacement of the stent- Optimal angiographic views with proximal positioning of stent marker
- The stent should be positioned protruding into the aorta by 1–2 mm to prevent recoil of the lesion at the stent edge
- Avoid using very short (<12 mm) stents to ensure adequate anchoring of the stent and to provide adequate lesion coverage distally
- Use the presence of ostial calcium to assist with stent positioning
- Stent pull-back technique for non aorto-ostial sites13
- Make sure to prepare lesion adequately, use of cutting balloon, rotational atherectomy is highly recommended
- Routine use of high pressure non-compliant balloon for post dilation
- Use of IVUS to assess for stent sizing and expansion is recommended
- Accurate stent positioning crucial
- If risk of side branch closure high (calcified, pre-existing lesion >50%, tortuous. lesion length >10mm) consider wire protection or upfront dedicated 2 stent bifurcation stenting if side branch size >2.5mm14
- Kissing PTCA to side branch if flow compromised
- IC vasodilators to exclude spasm
- Avoid deep intubation with guiding catheters or extension systems through already stented segment
- If resistance to passage of a secondary device in the stent do not push hard
- When LSD is suspected, radiographic assessment of the stented segment, preferably with StentBoost (Philips, Andover, Massachusetts) or an equivalent image-enhancement program, should be done
- Confirm wire position and use a small compliant balloon followed by a high-pressure noncompliant balloon aiming to ensure adequate expansion of deformed
stent struts and their apposition to coronary arterial. If LSD still persists, another stent can be used
- IVUS is strongly encouraged, although it is advisable to proceed to intracoronary imaging once LSD has been treated to avoid further potential deformation
- Adequate lesion preparation to prevent stent crossing failure
- Optimal angiographic views to accurately position the proximal stent marker
- Since the guide is disengaged during stent positioning, it may be difficult to visualize the ostia. If possible, use the presence of ostial calcium to assist with stent positioning
- Stent pull-back technique for non aorto-ostial sites13
- Use of buddy wire for positioning marker in side branch or aorta
- After stent deployment, perform light “flaring” of the ostium of the stent
- Use of Flash Ostial Balloon may be used for flaring of ostial stent15
- Adequate lesion preparation is key
- Avoid stent edge deployment at site of significant plaque (Imaging helpful)
- In case of dissection or perforation use prolonged balloon inflation +/- another stent deployment
References
- Brilakis E.S., Best P.J.M., Elesber A.A., et al. (2005) Incidence, retrieval methods, and outcomes of stent loss during percutaneous coronary intervention. Catheter Cardiovasc Interv 65:333–340.
- Bolte J., Neumann U., Pfafferott C., et al. (2001) Incidence, management, and outcome of stent loss during intracoronary stenting. Am J Cardiol 88:565–567.
- Kozman H., Wiseman A.H., Cook J.R. (2001) Long-term outcome following coronary stent embolization or misdeployment. Am J Cardiol 88:630–634.
- Francesco Giannini, Luciano Candilio, Satoru Mitomo, Neil Ruparelia, Alaide Chieffo, Luca Baldetti, Francesco Ponticelli, Azeem Latib, Antonio Colombo. Practical Approach to the Management of Complications During Percutaneous Coronary Intervention. J Am Coll Cardiol Intv. 2018 Sep, 11 (18) 1797-1810.
- Tan KH, Sulke N, Taub N, Sowton E. Percutaneous transluminal coronary angioplasty of aorta ostial, non-aorta ostial, and branch ostial stenoses: acute and long-term outcome. Eur Heart J 1995;16:631-9.
- Mavromatis K, Ghazzal Z, Veledar E, Diamandopoulos L, Weintraub WS, Douglas JS, Kalynych AM. Comparison of outcomes of percutaneous coronary intervention of ostial versus nonostial narrowing of the major epicardial coronary arteries. Am J Cardiol 2004;94:583-7.
- Jokhi P, Curzen N. Percutaneous coronary intervention of ostial lesions. EuroIntervention 2009;5:511-514.
- Rocha-Singh K, Morris N, Wong SC, Schatz RA, Teirstein PS. Coronary stenting for treatment of ostial stenoses of native coronary arteries or aortocoronary saphenous venous grafts. Am J Cardiol 1995;75:26-9.
- Iakovou I, Ge L, Michev I, Sangiorgi GM, Montorfano M, Airoldi F, Chieffo A, Stankovic G, Vitrella G, Carlino M, Corvaja N, Briguori C, Colombo A. Clinical and angiographic outcome after sirolimus-eluting stent implantation in aorto-ostial lesions. J Am Coll Cardiol 2004;44:967-71.
- Dunning DW, Kahn JK, Hawkins ET, O'Neill WW. Iatrogenic coronary artery dissections extending into and involving the aortic root. Catheter Cardiovasc Interv. 2000;51(4):387–393.
- Boukhris M, Tomasello SD, Marzà F, Azzarelli S, Galassi AR. Iatrogenic aortic dissection complicating percutaneous coronary intervention for chronic total occlusion. Can J Cardiol. 2015;31(3):320–327.
- Wykrzykowska JJ, Ligthart J, Lopez NG, Schultz C, Garcia-Garcia H, Serruys PW. How should I treat an iatrogenic aortic dissection as a complication of complex PCI? EuroIntervention. 2012;7(9):1111–1117.
- Kini AS, Moreno PR, Steinheimer AM, et al. Effectiveness of the stent pull-back technique for nonaorto ostial coronary narrowings. Am J Cardiol. 2005;96(8):1123-1128. doi:10.1016/j.amjcard.2005.06.043.
- Sharma SK, Sweeny J, Kini AS. Coronary bifurcation lesions: a current update. Cardiol Clin. 2010;28(1):55‐70. doi:10.1016/j.ccl.2009.10.001.
- Riley R, Lombardi B. Solving the dilemma of ostial stenting: a case series illustrating the flash ostial system.Cardiol Cardiovascmed. 2017;1(1):64-71.
BYPASS GRAFT COMPLICATIONS
Coronary artery bypass graft procedure is usually performed in severe three-vessel disease or two vessels with a left main disease using arterial or vein grafts. The grafts' longevity varies depending on many factors, including the quality of the grafts, underlying comorbidities, and surgical techniques. However, saphenous vein grafts (SVGs) are prone to degeneration and occlusion, leading to poor long-term patency compared with arterial grafts with ~ 10−25% of SVGs failure within the first year after CABG surgery 1,2 and late graft failure at ≥ten years is 40 -50%.3 Hence, PCI of the native artery should be the preferred
Common Complications associated with percutaneous intervention of bypass grafts are:
- Slow flow/ no-reflow
- Peri-procedural myocardial infarction
Slow flow/No-reflow
It is a common complication of degenerative graft intervention and can be preventable/treatable if the appropriate treatment steps are followed.
Incidence: 10-15% cases of percutaneous intervention (PCI) in aortocoronary saphenous vein grafts (SVG).
- Optimal anticoagulant and antiplatelet therapy
- Ensure to obtain optimal guiding-catheter support
- Consider aspiration thrombectomy in a setting of heavy thrombus burden
- Avoid pre and post dilation if possible
- Direct stenting strategy if feasible
- Use of embolic protection devices(EPD) whenever technically feasible
- Ensure to keep the optimal blood pressure (SBP > 100Hg) and give fluids, vasopressors, and pacing as needed
- Administer intracoronary vasodilators, such as adenosine, nitroprusside, nitroglycerin, nicardipine, and verapamil administered distally in the vessel if necessary, via a dual-lumen microcatheter (Twin-pass catheter)
- If EPD is used, consider to use aspiration thrombectomy to clear debris from the filter first, then remove the filter if necessary. New filter can be placed if needed.
- If hemodynamically unstable, place an intra-aortic balloon pump to augment coronary perfusion pressure
Peri-procedural Myocardial infarction
The risk of developing CK-MB elevation is relatively higher during saphenous vein graft (SVG) than native coronary intervention, probable due to more friable atherosclerotic or thrombotic components of the SVG lesions.5
Incidence: 15% of patients who underwent SVG intervention were found to have CK-MB levels >5x the upper limit of normal (ULN).6
- Avoid pre-dilation with balloon angioplasty
- Use Direct stenting strategy whenever possible (direct stenting were associated with nearly a 50 % reduction in CK-MB level elevations >4x normal)7
- Appropriate stent sizing
- Avoid high pressure balloon inflation with maximum inflation pressures of 12-14 atm.
- Adequate use of anticoagulant and antiplatelet therapy
- Use of Embolic protection devices whenever possible
- Use of post PCI optimal antiplatelet therapy (Dual antiplatelets) if there is no contraindication
- Use of coronary vasodilators if there is slow flow/no-reflow phenomenon
- Consider to use oral coronary vasodilators and beta blocker if residual chest pain presents
- Optimize medical therapy for underlying comorbidities
References
- Hess, C. N. et al. Saphenous vein graft failure after coronary artery bypass surgery: insights from PREVENT IV. Circulation 130, 1445–1451 (2014).
- Fitzgibbon, G. M. et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J. Am. Coll. Cardiol. 28, 616–626 (1996).
- Caliskan E, de Souza DR, Böning A, et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat Rev Cardiol. 2020;17(3):155-169. doi:10.1038/s41569-019-0249-3
- Piana RN, Paik GY, Moscucci M, Cohen DJ, Gibson CM, Kugel-mass AD, Carrozza JP Jr, Kuntz RE, Baim DS. Incidence and treatment of “no-reflow” after percutaneous coronary intervention.Circulation 1994;89:2514–2518.
- Abdelmeguid AE, Topol EJ, Whitlow PL, Sapp SK, Ellis SG. Significance of mild transient release of creatine kinase-MB fraction after percutaneous coronary interventions. Circulation.1996; 94:1528–1536.
- Hong MK, Mehran R, Dangas G, et al. Creatine kinase-MB enzyme elevation following successful saphenous vein graft intervention is associated with late mortality. Circulation 1999;100:2400–5.
- Leborgne L, Cheneau E, Pichard A, et al. Effect of direct stenting on clinical outcome in patients treated with percutaneous coronary intervention on saphenous vein graft. Am Heart J 2003;146:501–6.