Acute Stent Thrombosis – Case 1
Clinical Presentation
- 63-year-old male who presented with chest pain (CCS Class 3), and was referred for PCI of the LM trifurcation.
Past Medical History
- HTN, HLD, DM, Former Tobacco Use, CAD s/p 3-Vessel CABG and Multiple PCI’s, ESRD on iHD, PVD s/p Fem-Pop Bypass and Bilateral Toe Amputations, BPH
- LVEF 53%
Clinical Variables
- Stress MPI: Mild anterolateral ischemia and moderate posterior scarring.
- Prior Cardiac Catheterization: Ostial LM 70-80%, distal LM 60-70% stenosis, proximal LAD 70-80% stenosis, D1 90% stenosis, OM1 total occlusion and fills via SVG, LPL 60-70% stenosis, proximal RI 70-80% stenosis, proximal RCA 80-90% stenosis and fills retrograde via SVG; SVG-Y graft to RPDA and OM1 (patent), LIMA to LAD known to be occluded.
Medications
- Home Medications: Aspirin, Clopidogrel, Rosuvastatin, Carvedilol, Valsartan-Hydrochlorothiazide, Isosorbide Monnitrate, Clonidine, Doxazosin, Insulin, Calcium Acetate, Calcitriol, Epoetin-Alpha
- Adjunct Pharmacotherapy: Clopidogrel, Bivalirudin
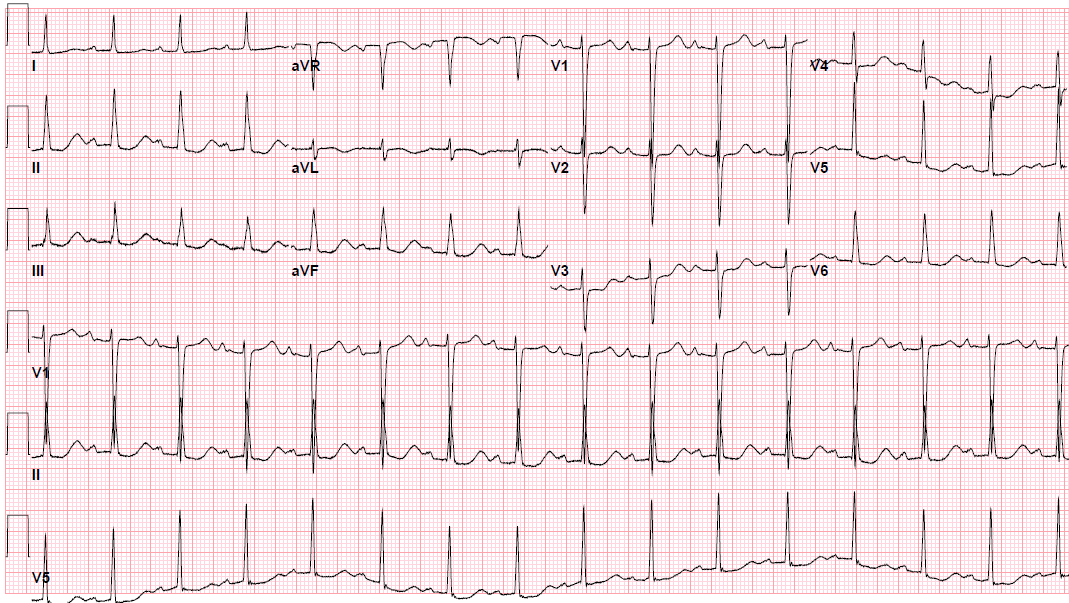
Pre-procedure EKG
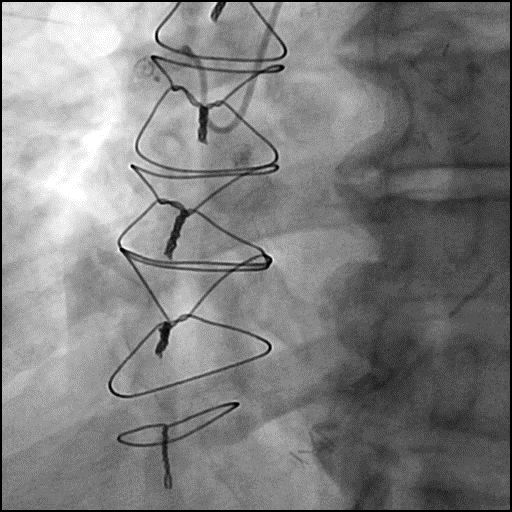
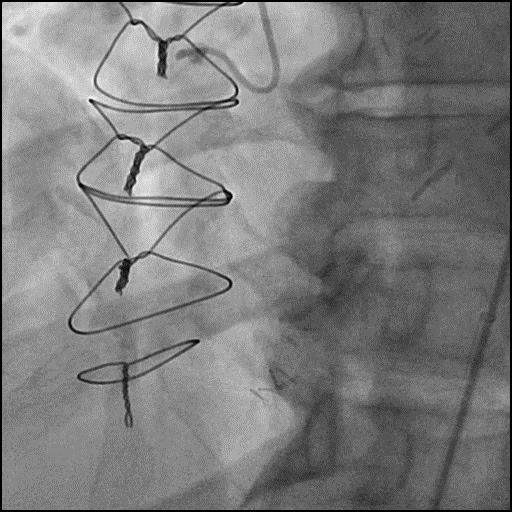
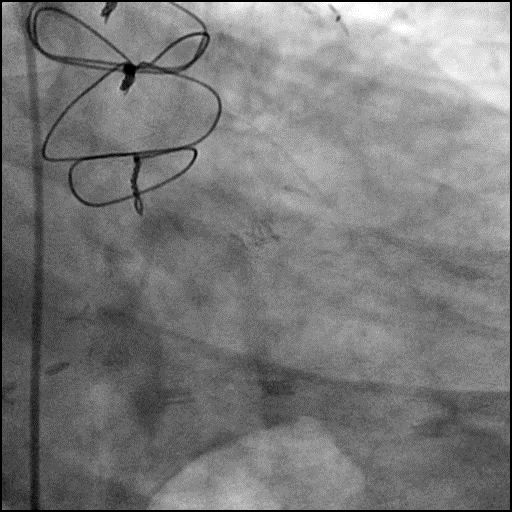
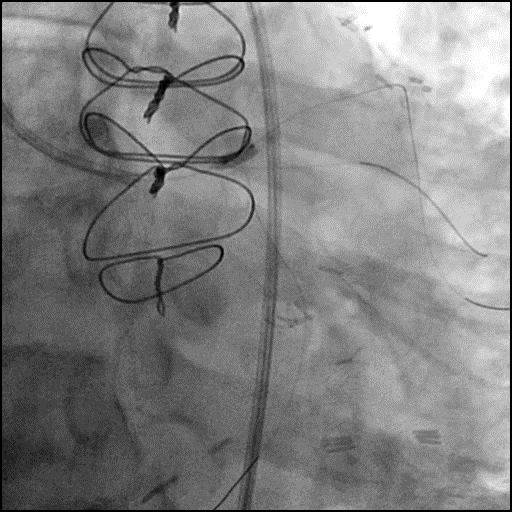
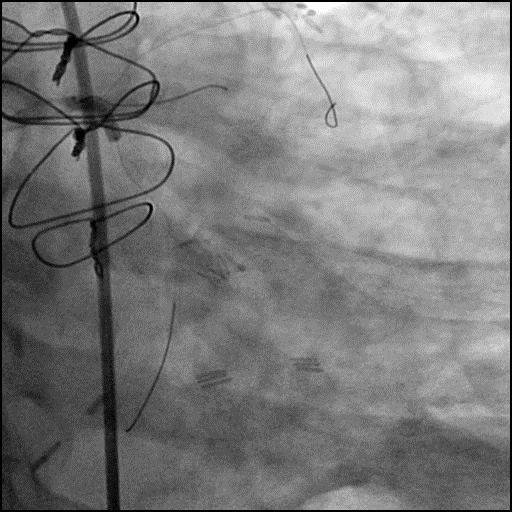
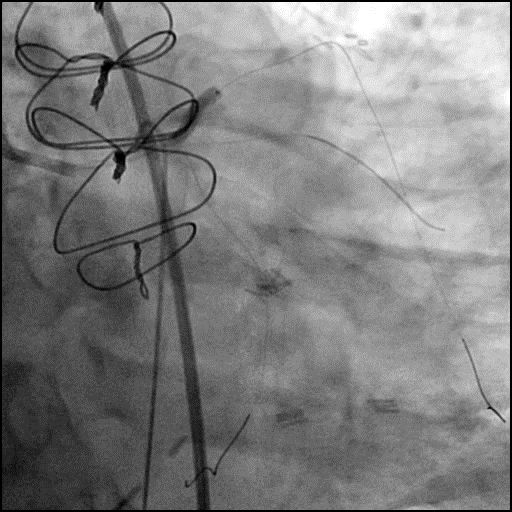
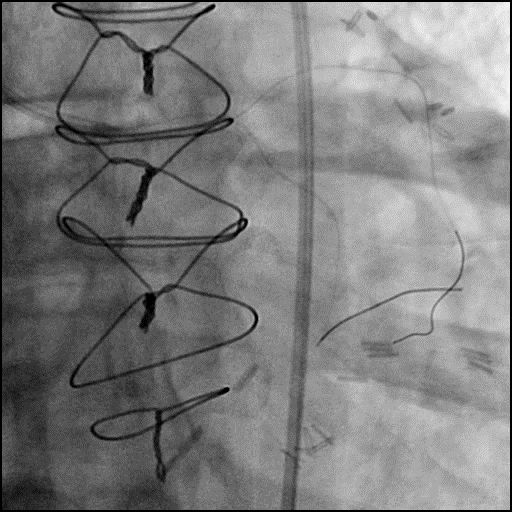
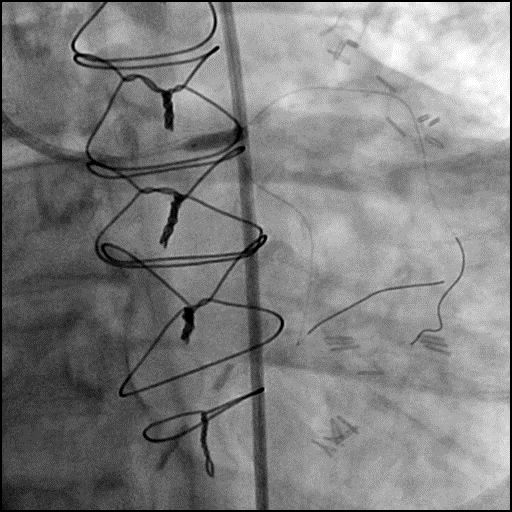
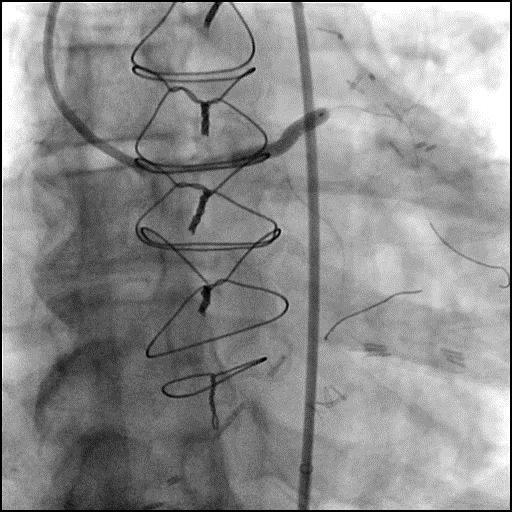
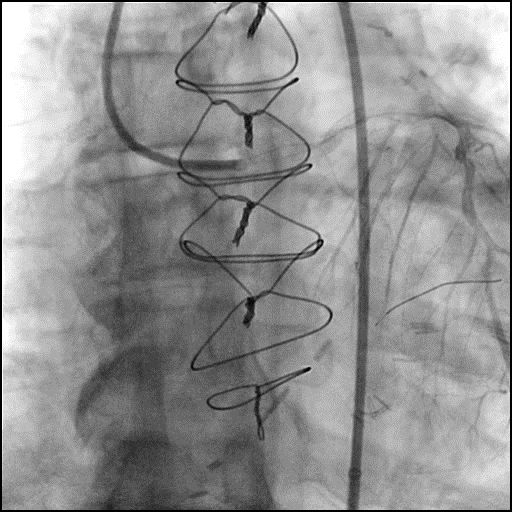
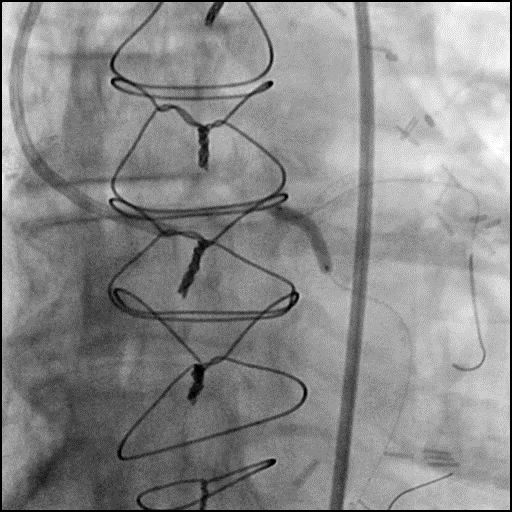
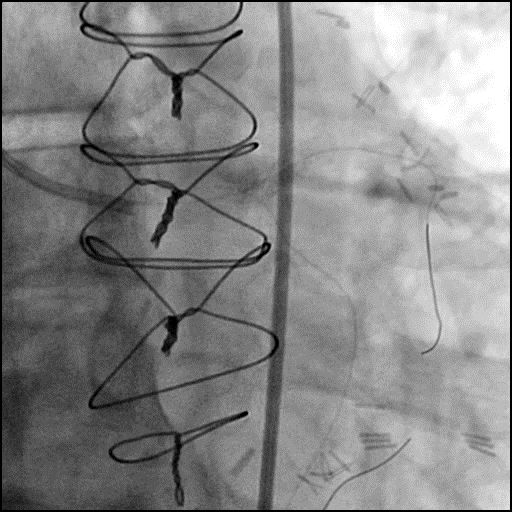
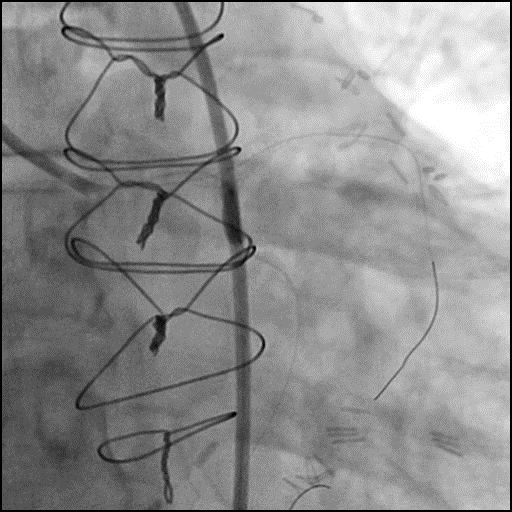
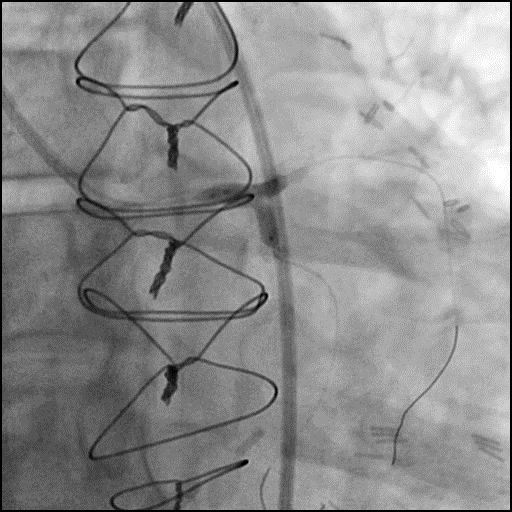
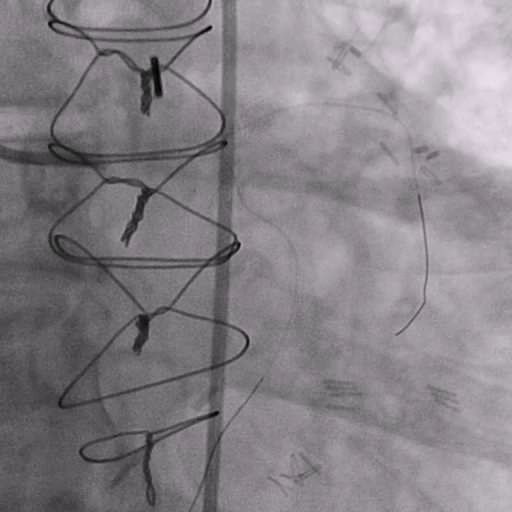
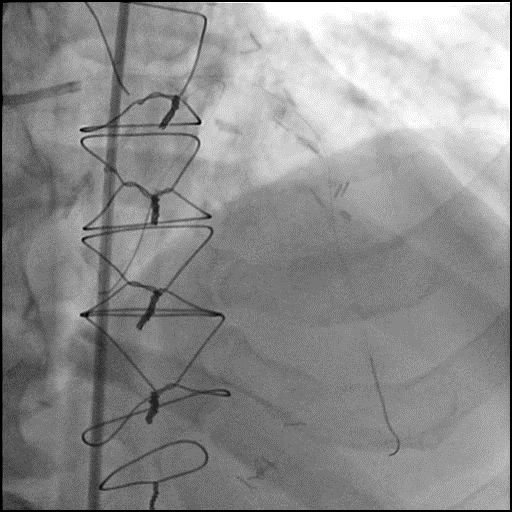
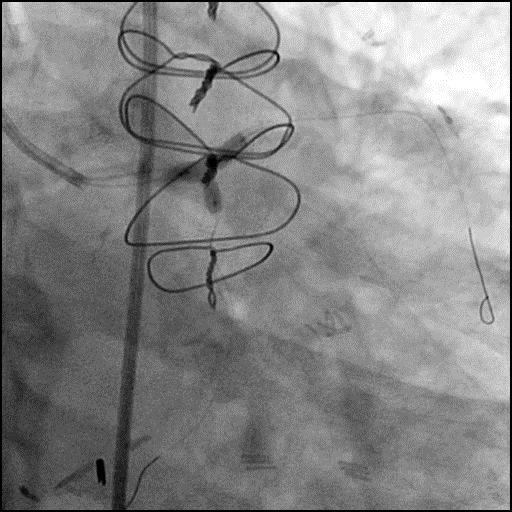
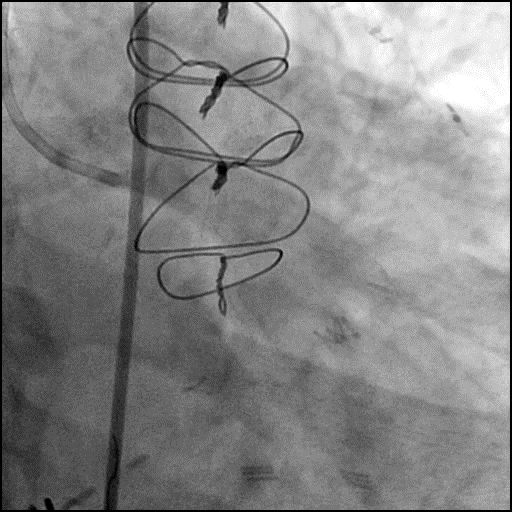
Angiograms
Post-procedure EKG
Case Overview
- Underwent complex intervention of LM trifurcation.
- Procedure was complicated by abrupt vessel closure (AVC) of the LM likely due to acute thrombus/Type F dissection following lesion pre-dilatation.
- Patient acutely decompensated and an IABP was emergently placed.
- Subsequently, patient developed ventricular tachycardia followed by ventricular fibrillation which was successful treated with a single debrillator shock of 200 J.
- Serial balloon inflations of the LM performed without improvement in flow.
- Procedure was continued and a stent was placed in the LM extending into the proximal LAD.
- Follow up angiography showed successful restoration of flow in the LM-LAD; however, the procedure was further complicated by stent jailing of the RI and LCx.
- PTCA of the ostial LCx was performed, restoring flow in the LCx.
- Next, a stent was successfully placed in the LCx. This was followed by KBI of the LM-LAD and LCx.
- However, procedure was now complicated by embolization of thrombus to the distal LAD.
- A wire was used to traverse the distal LAD thrombus, successfully restoring flow in the distal LAD.
- PTCA of the stent jailed RI was performed, but unsuccessful.
- Troponin-I peaked at 38.2 ng/mL and CK-MB peaked at 16.9 ng/mL.
- Patient was discharged home two days later without further sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- Abrupt vessel closure (AVC) due to acute thrombosis/Type F dissection following lesion pre-dilatation.
- How could the complication have been prevented?
- Assure patient is given adequate periprocedure antithrombotic therapy (antiplatelets and anticoagulants).
- Pay close attention to the ACT during the procedure and dose anticoagulation accordingly to maintain ACT >300 prior to performing an intervention (Hemochron machine).
- Avoid aggressive, high pressure, over sized balloon dilatation of a calcified lesion as this can result in complications.
- Avoid placement of multiple wires (≥3 wires) within the coronary system simultaneously because this increases the risk for thrombosis within the guide catheter and coronary arteries.
- Lesions preparation with atherectomy (rota, orbital or laser) prior to performing lesion pre-dilatation could have helped reduce calcified plaque burden, and made it safer to prepare and reduce the chance of dissection.
- Is there an alternate strategy that could have been used to manage the complication?
- Intravascular imaging of the coronaries with IVUS would have helped with determining the etiology of AVC, and guide further management but given the certainty of AVC being due to acute thrombosis/Type F dissection the complication was immediately treated with PTCA followed by placement of a stent.
- What are the important learning points?
- When AVC occurs after PTCA or stent placement you have to consider a broad differential which includes the possibility of a coronary spasm, thrombus or debris with distal embolization, and dissection.
- A stent should not be placed prior to restoration of flow in the vessel with AVC unless you are confident the complication is due to a dissection. Generally, a stent should only be placed after flow has been restored in a vessel which has AVC.
- In this case, following pre-dilatation of a calcified lesion there was AVC of the LM due to acute thrombosis/Type F dissection. Intracoronary vasodilators were given with no improvement in flow. We were confident the cause for AVC was due to acute thrombosis/Type F dissection, and PTCA was performed as a bridge with no improvement in flow. A stent was subsequently placed with immediate improvement in flow noted, confirming our suspicion for acute thrombosis/Type F dissection. However, the procedure was further complicated by stent jailing of the LCx and RI. Alternatively, if our concern for AVC was due to thrombus without dissection, we could have performed aspiration thrombectomy prior to placement of a stent.
- If you are performing stenting with side branch wiring, and the side branch is stent jailed with the wire still in position, it is imperative you do not remove that wire. Instead, place a second wire across the vessel which is stent jailed, and then remove the wire. If this is not feasible, use a 1.0 compliant balloon and advance it over the wire which is stent jailed and wedge it at the ostium of the stent and remove the wire. Next, introduce another wire across this vessel and continue with the procedure.