Dissection Type B – Case 1
Clinical Presentation
- 83-year-old female who presented with chest pain (CCS Class III) associated with dyspnea. Underwent cardiac catheterization which showed 2-Vessel CAD with LM involvement. She was subsequently referred for CABG but she declined in favor of percutaneous revascularization. Subsequently, she was referred for PCI of LM bifurcation.
Past Medical History
- HTN, HLD, CAD, DVT/PE (2009)
- LVEF 60%
Clinical Variables
- Cardiac CTA: Severe 3-Vessel CAD.
- Prior Cardiac Catheterization: Distal LM 60-70% stenosis, proximal LAD 70-80% stenosis, and proximal LCx 80-90% stenosis with severe calcification of the vessels.
Medications
- Home Medications: Home medications: Aspirin, Clopidogrel, Simvastatin, Ezetimibe, Fenofibrate, Atenolol, Amlodipine, Ranolazine
- Adjunct Pharmacotherapy: Clopidogrel, Ticagrelor, Bivalirudin
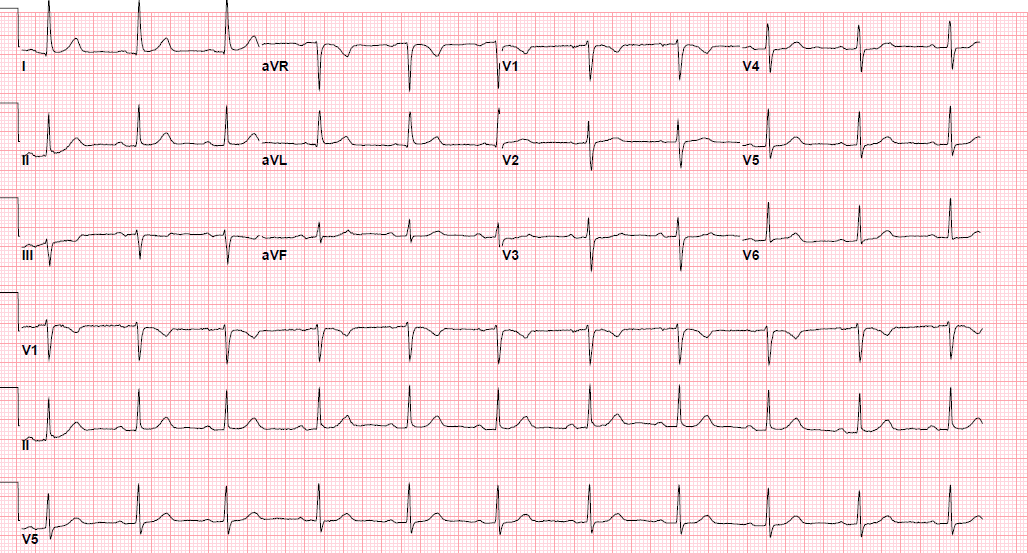
Pre-procedure EKG
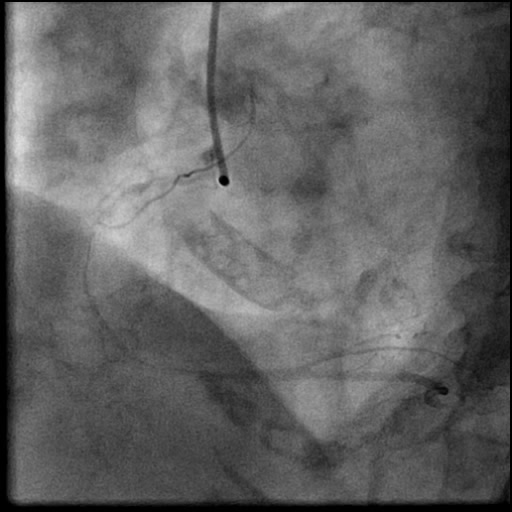
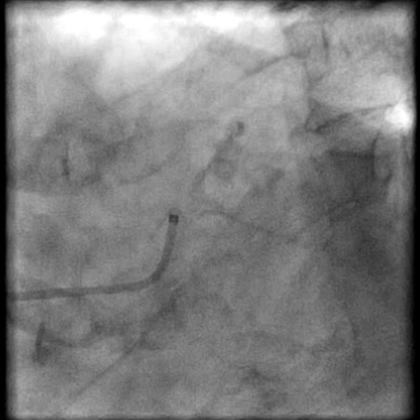
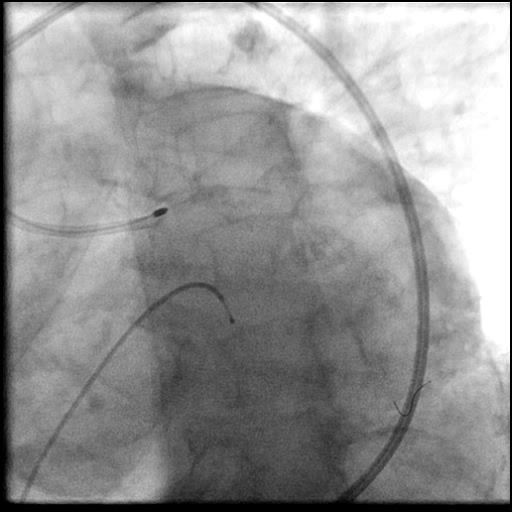
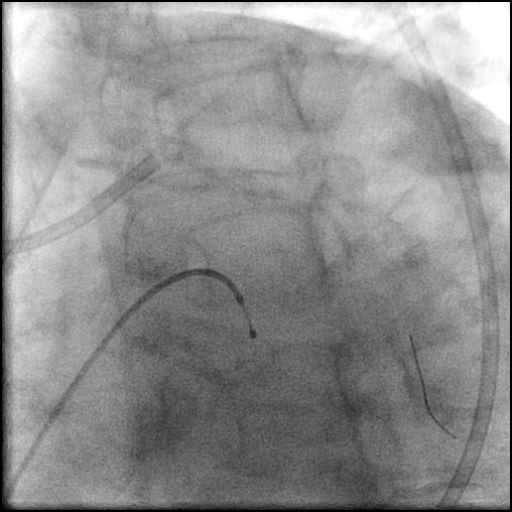
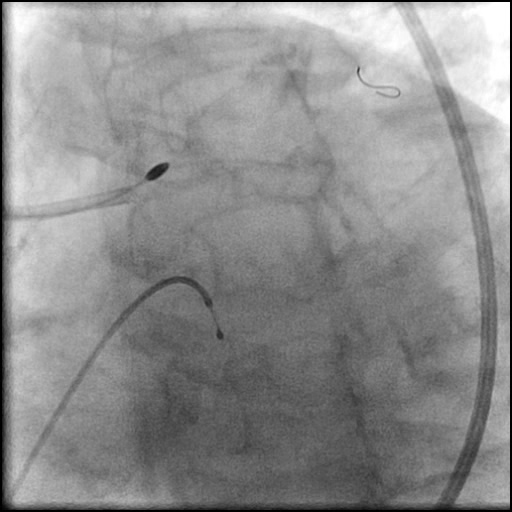
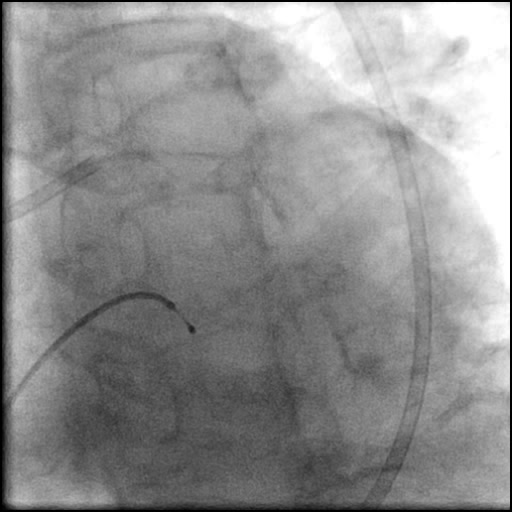
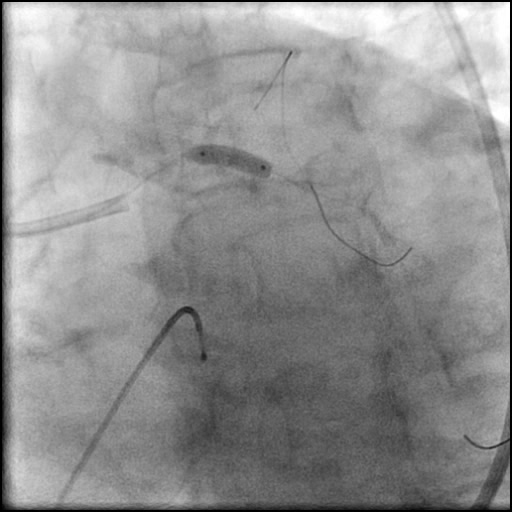
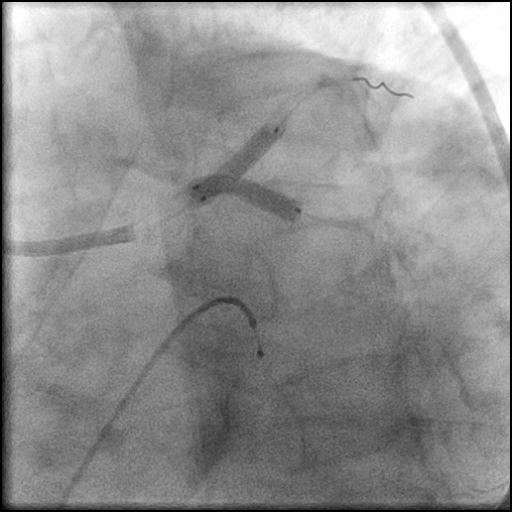
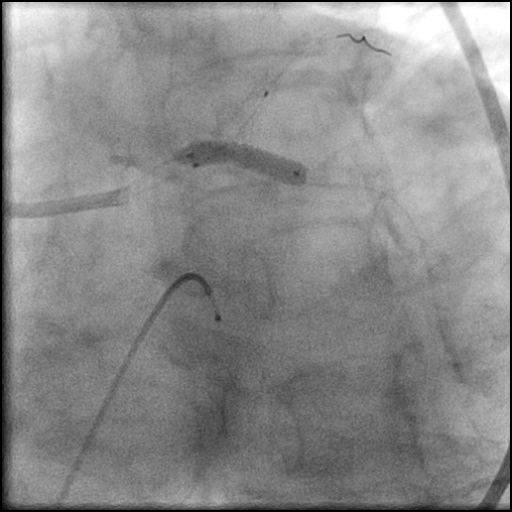
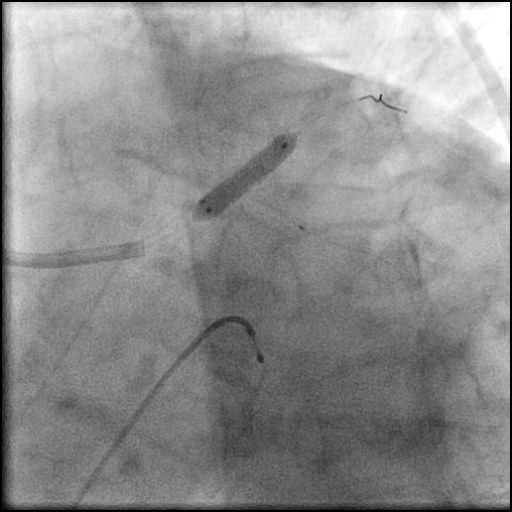
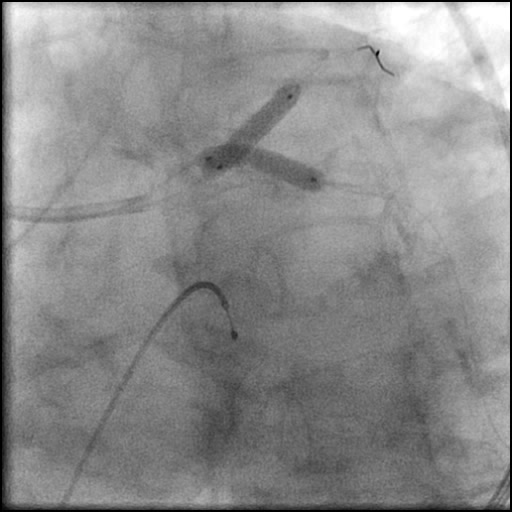
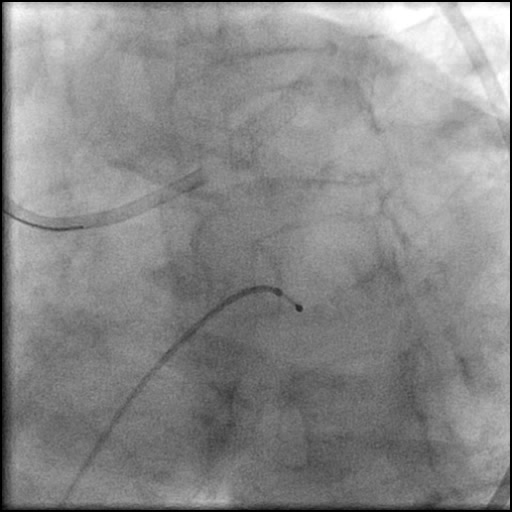
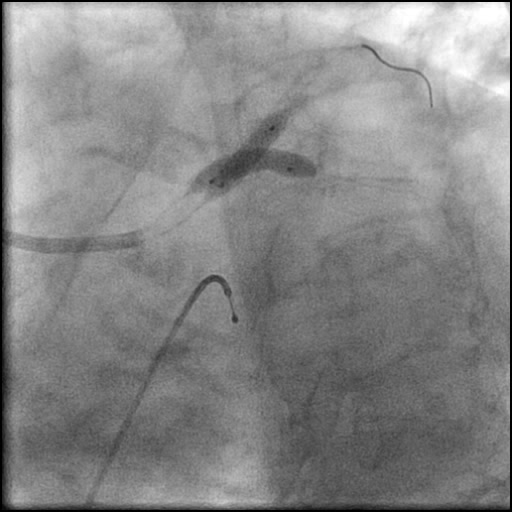
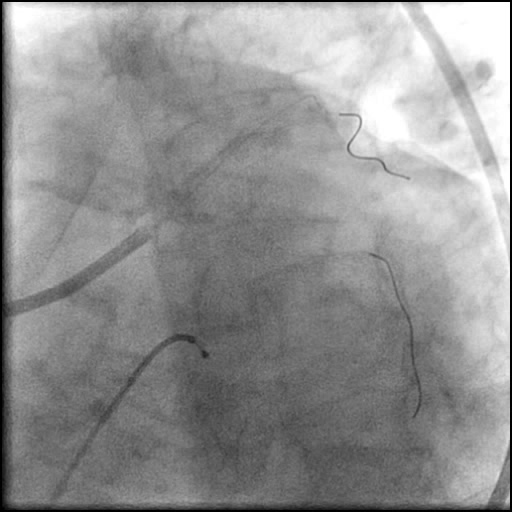
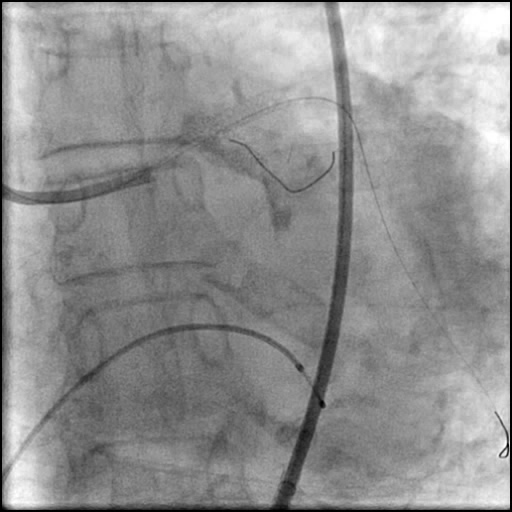
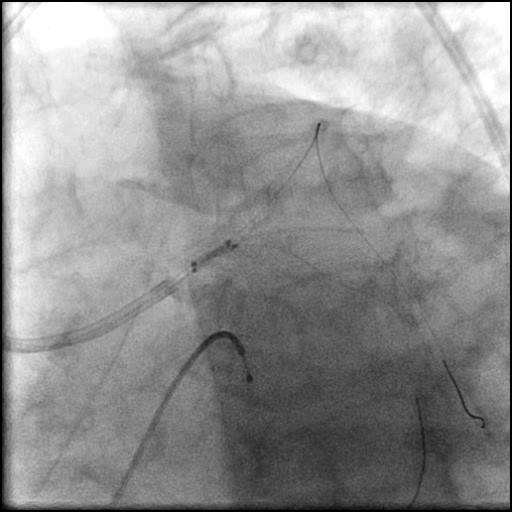
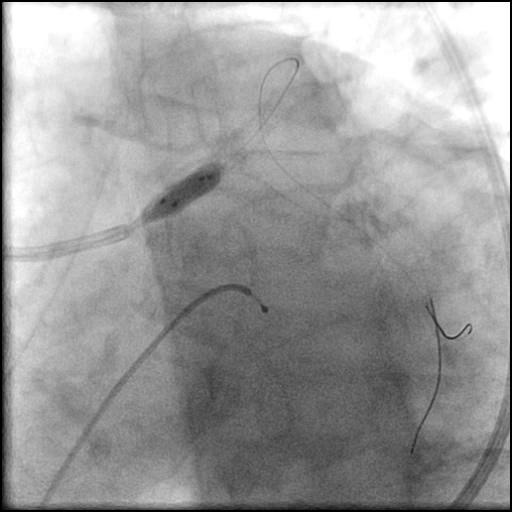
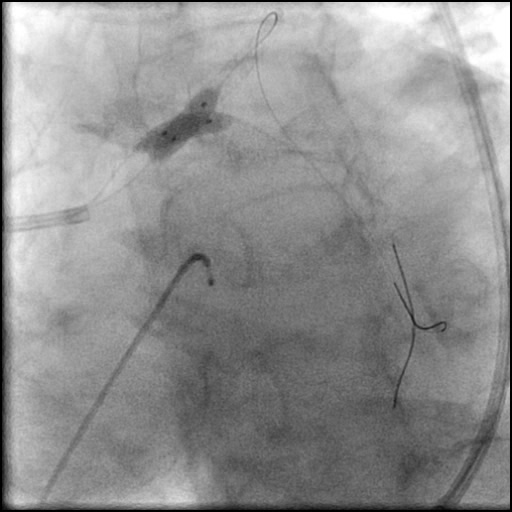
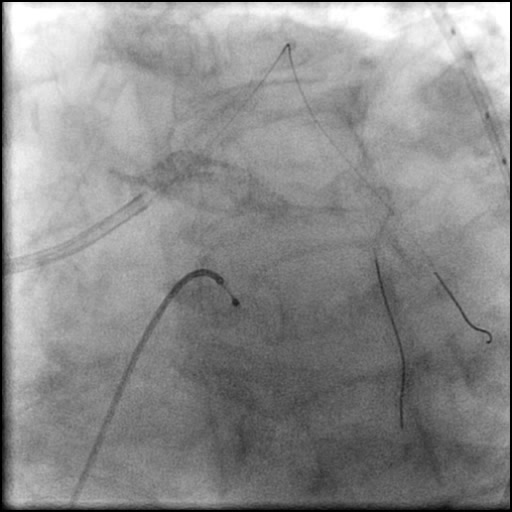
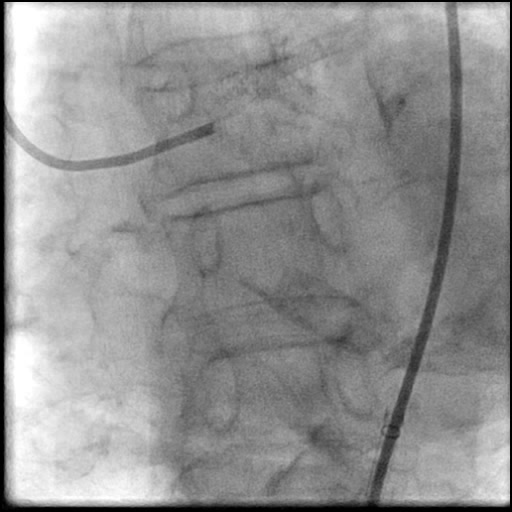
Angiograms
Post-procedure EKG
Case Overview
- Underwent OCT guided PCI of the distal LM bifurcation (distal LM MLA of 5.1 mm²).
- SKS technique was used to place a stent in the LM bifurcation.
- Procedure was complicated by a non-flow limiting, Type B dissection of the LM proximal to the newly placed stents.
- Two stents were placed in a ‘parallel, double barrel’ manner overlapping into the previously placed stents in the LM.
- Follow up angiography revealed, inadequate treatment of the dissection with incomplete coverage of the dissection flap.
- Patient was observed on the catheterization table and repeat angiography showed no change in the residual dissection.
- Post intervention OCT showed LM MLA 10.9 mm² with residual minor proximal edge dissection (residual dissection was < 2 mm in length). Therefore, further intervention was deferred.
- Troponin-I peaked at 0.4 ng/mL and CK-MB peaked at 2.6 ng/mL.
- Patient was discharged the next day without further sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- High pressure post-dilatation of the proximal stent edge.
- How could the complication have been prevented?
- Optimize balloon position and assure it is not outside the proximal or distal stent edge prior to balloon inflation.
- Post-dilate the stent edges using a lower balloon inflation pressure.
- When performing SKS, KBI maximal pressure dilatation of the stent balloons is 12 atm simultaneously.
- When performing individual dilatation of the stents after SKS are placed, the maximal pressure to inflate the stent balloons is 16 atm.
- Consider using separate shorter NC balloons instead of the stent balloons after SKS were deployed.
- Is there an alternate strategy that could have been used to manage the complication?
- Consider an alternative stenting technique to treat the dissected segment such as reverse crush.
- Convert SKS into crush technique by crushing the LM-LCx stent with a balloon placed inside the LM-LAD stent, followed by recrossing of LCx and performing final KBI.
- What are the important learning points?
- This is a type B dissection because of the presence of a parallel tract linear defect seen during contrast injection.
- Maintaining wire position is extremely important, especially when dealing with complex interventions. Be cautious to avoid loss of wire position and possible wire entrapment.
- Guide catheter induced dissection should always be considered in the differential when there is a LM dissection.
- The larger the size of the guide catheter the higher the risk for guide catheter induced dissection. We used a large sized guide catheter (8 Fr) to accommodate the large size rota burr (2.15 mm).
- Optimize post dilatation technique.
- Use a noncompliant, short balloon for post-dilatation of a stent.
- Keep the balloon just inside the stent edge when performing post-dilatation of the proximal or distal stent edges.
- Use Stent Boost (Philips) or Stent Viz (GE) to assess the positioning of the NC balloon prior to inflation.
- Post intervention imaging with OCT was performed and the dissection length was < 2 mm. Conservative management is appropriate if the dissection length is < 2 mm and dissection is not propagating.
- The patient remained in the cardiac catheterization lab for 30 minutes. Repeat angiography was performed and dissection was stable; therefore, we opted for conservative management instead of performing further intervention.
- Note: SKS technique is now rarely being used.