No Reflow – Case 4
Clinical Presentation
- 70-year-old female who presented with chest pain (CCS Class III).
Past Medical History
- HTN, HLD, DM, Former Tobacco Use, CAD s/p Multiple PCI’s, CVA, OSA, , IBS, UC
- LVEF 56%
Clinical Variables
- MPI: Moderate anteroseptal ischemia.
Medications
- Home Medications: Aspirin, Clopidogrel, Ezetimibe, Diltiazem, Quinapril, Furosemide, Albuterol, Sitagliptan, Insulin, Ranitidine
- Adjunct Pharmacotherapy: Clopidogrel, Ticagrelor, Bivalirudin
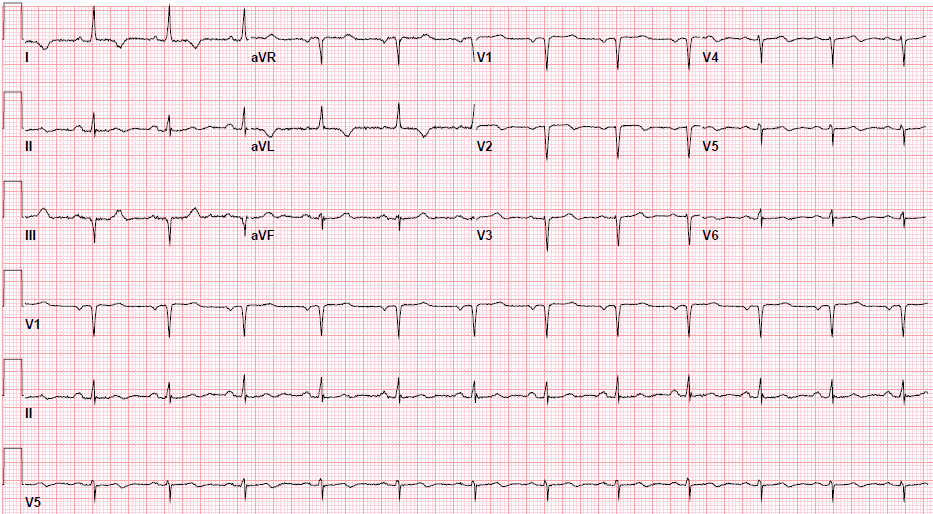
Pre-procedure EKG
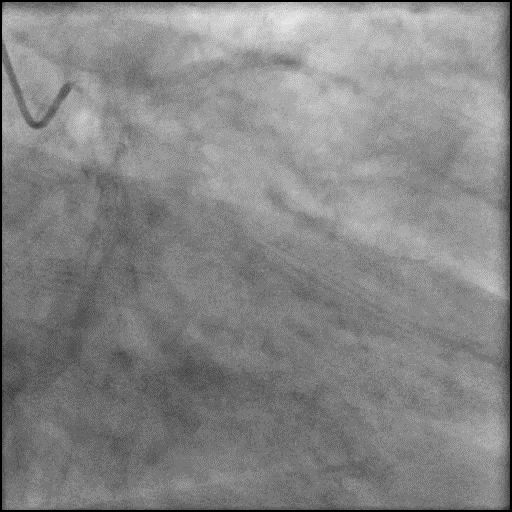
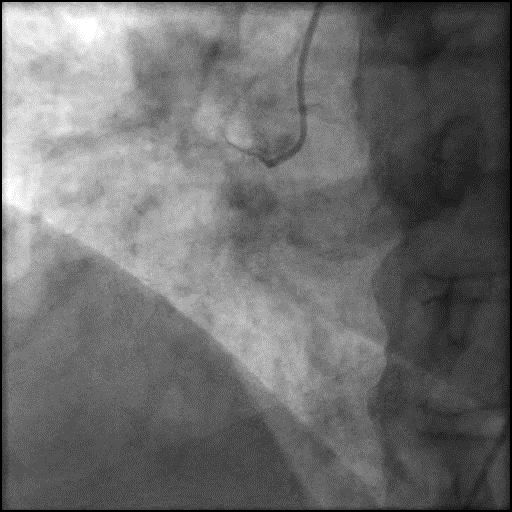
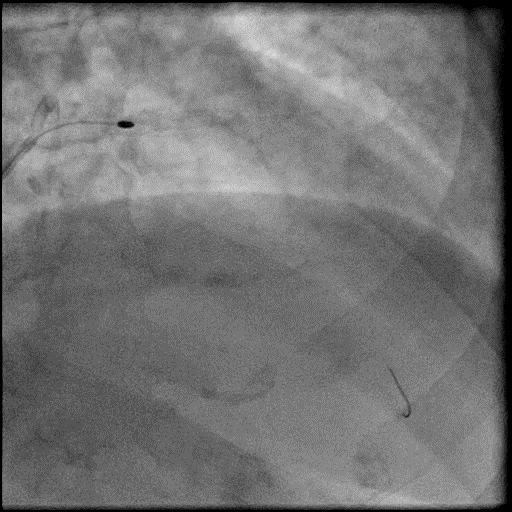
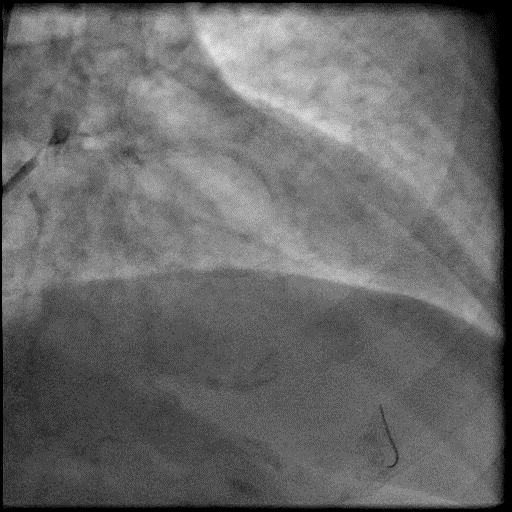
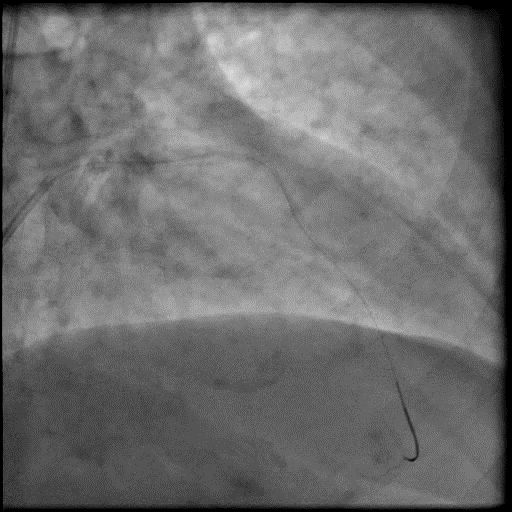
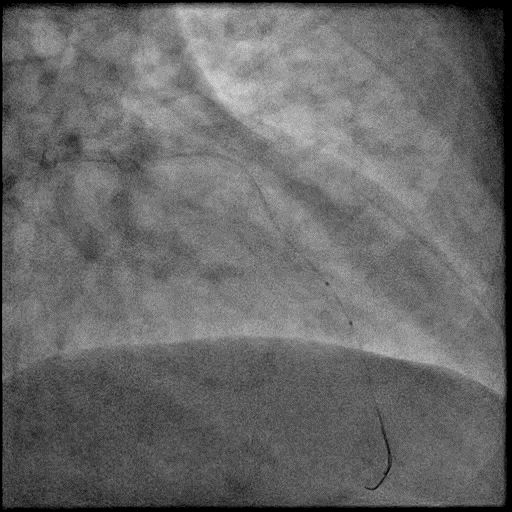
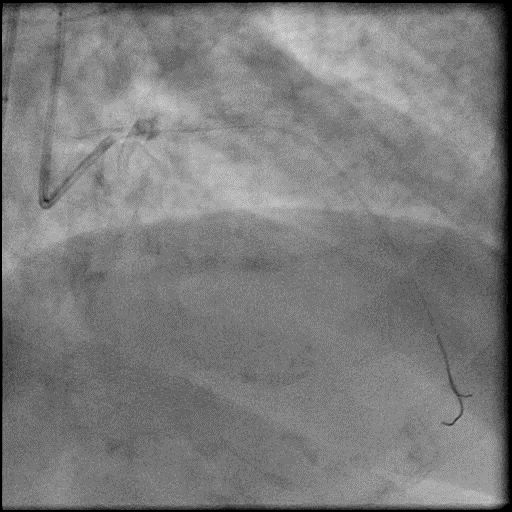
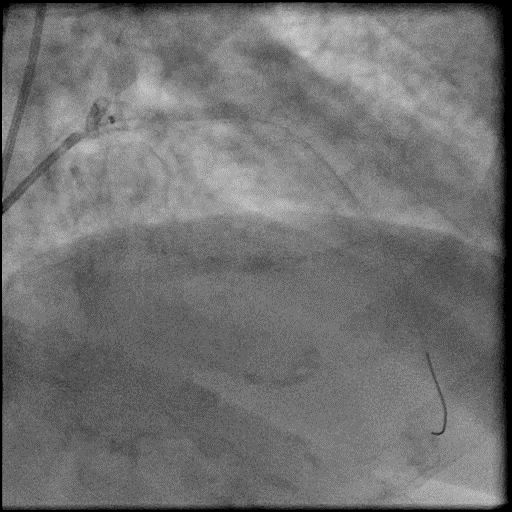
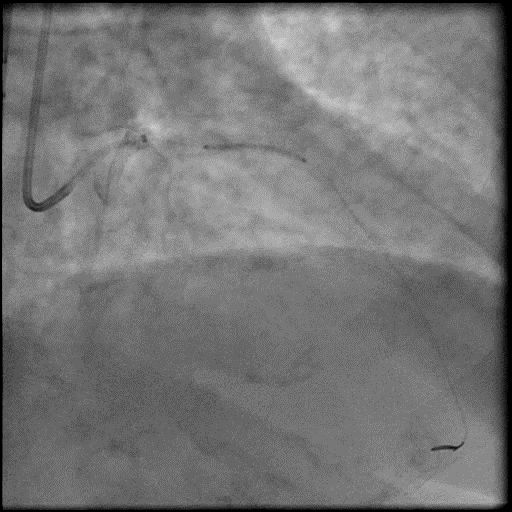
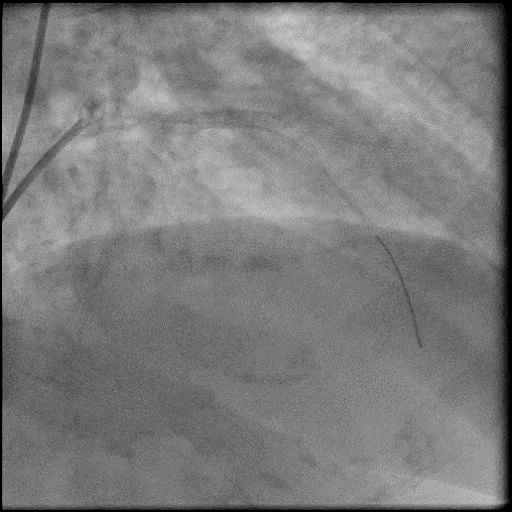
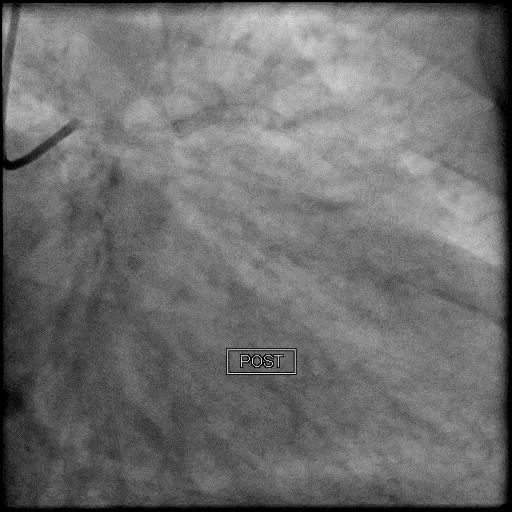
Angiograms
Post-procedure EKG
Case Overview
- Underwent intervention of the LAD.
- Procedure was complicated by abrupt vessel closure (AVC) due to no-reflow after rotation atherectomy.
- IC vasodilators administration through the guide catheter with no improvement in flow.
- Microcatheter was used to inject contrast into the distal vessel. Retrograde flow was preserved and distal flow was absent confirming our suspicion that AVC was likely due to distal embolization of debris following rotational atherectomy.
- Microcatheter was then used to administer IC vasodilators to the distal vessel and microvasculature, resulting in improved flow.
- After restoring flow in the LAD, the procedure was continued and a stent was placed in the mid LAD.
- Troponin-I peaked at 29.14 ng/mL and CK-MB peaked at 174.9 ng/mL.
- Patient was discharged 2 days later without sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- No-reflow flow following rotational atherectomy – likely due to dissection.
- How could the complication have been prevented?
- When performing rotational atherectomy consider the following to prevent complications:
- Keep systolic BP >100mmHg and avoid bradycardia
- Perform short runs <20 seconds with a gentle pecking motion to advance the rota burr, maintain rotational speed around 150-160k RPM
- Use Rota-flush and consider use of glycoprotein IIB/IIIA inhibitors
- Vasodilators should always be given prophylactically and for treatment of slow flow/no-reflow.
- Assure patient is given adequate periprocedure antithrombotic therapy (antiplatelets and anticoagulants).
- Pay close attention to the ACT during the procedure and dose anticoagulation accordingly to maintain ACT >300 prior to performing an intervention (Hemochron machine).
- Use a rota-burr which is appropriately sized for the vessel. Ideally the burr should be 0.5-0.6x the distal vessel size when performing a plaque modification strategy, and 0.8-0.85x the distal vessel size when performing a debulking strategy (STRATA trial). With plaque modification strategy the purpose is to disrupt the calcium so it allows for safe passage of devices and easier expansion of device, balloons, and stents. With debulking, the aim is to break the calcium into particles so it can move through the coronary artery and eventually washout of the coronary microcirculation.
- Lithotripsy device: Currently under investigation in the USA (approved for use in Europe). It is uncertain if this device will result in reduced complications including slow flow.
- Is there an alternate strategy that could have been used to manage the complication?
- The initial step in management of slow flow/no-reflow involves administration of intra-coronary vasodilators through the guide catheter. If this fails, recommended using a dual-lumen microcatheter (Twinpass is the only dual lumen microcatheter available in the USA) to deliver intra-coronary medications to the distal vessel and microvasculature. Next, perform angiography with delivery of contrast through the microcatheter to determine if there is distal coronary flow. If distal vessel flow is not preserved the likely etiology of abrupt vessel closure (AVC) is no-reflow due to distal embolization of debris or thrombus, and IC vasodilators should be administered through the microcatheter targeted to the distal vessel and microvasculature. If flow is preserved, then the likely etiology of AVC is dissection (proximal to the point of microcatheter injection), and treatment involves placement of a stent. It is reasonable to perform aspiration thrombectomy prior to microcatheter based angiography injection (depending on the clinical context/presence of thrombus).
- Intravascular imaging of the coronaries with IVUS would have helped with determining the etiology of AVC, and guide further management.
- What are the important learning points?
- The best treatment for slow flow/no-reflow is to prevent it from happening.
- The exact mechanism of the no-reflow phenomenon is unclear, but it is thought to be associated with endothelial swelling, neutrophil infiltration, and platelet aggregation causing obstruction and spasm in the microvasculature.
- One of the possible complications of PCI especially during rotational or orbital atherectomy is slow flow/no-reflow. The common settings where this complication arises are long calcified lesions, CTO (particularily RCA CTO), thrombotic lesions, vein graft intervention, poor LV function and hemodynamic instability.
- Important to have multiple vasodilators readily available during a procedure. We use the following agents and administer them intra-coronary:
- Nitroprusside 50-200 mcg, Adenosine 30-40 mcg, Verapamil 100-200 mcg, Nicardipine 100-200 mcg
- Nitroglycerin 100-200 mcg (we use NTG for slow flow/no-reflow when it involved the epicardial vessels and not the coronary microvasculature)
- If the patient is hypotensive and this impedes the administration of intra-coronary vasodilators to treat slow flow/no-reflow, we recommend administration of IV phenylephrine 100-200 mcg as needed (may result in reflex bradycardia) to increase blood pressure, and then administer intra-coronary vasodilators.
- If there is refractory slow flow/no reflow then consider placement of an IABP. This helps with reduction in afterload, and improves coronary perfusion pressure by increasing coronary blood flow during diastole, and reduction in LVEDP.
- Depending on the size and function of the LV (LVEF <30%), consider using upfront LV support as it can help improve coronary perfusion pressure.
- Distal flow should always be established before placing a stent, unless dissection is considered as an etiology.