Perforation Type 2 Device – Case 1
Clinical Presentation
- 82-year-old male who presented with chest pain (CCS Class III).
Past Medical History
- HTN, HLD, Former Tobacco Use, Asthma
- LVEF unknown
Clinical Variables
- ETT: Abnormal. High risk.
- Prior Cardiac Catheterization: LAD FFR = 0.75.
Medications Heading
- Home Medications: Rosuvastatin, Amlodipine, Valsartan, HCTZ, Potassium Chloride
- Adjunct Pharmacotherapy: Clopidogrel, Bivalirudin
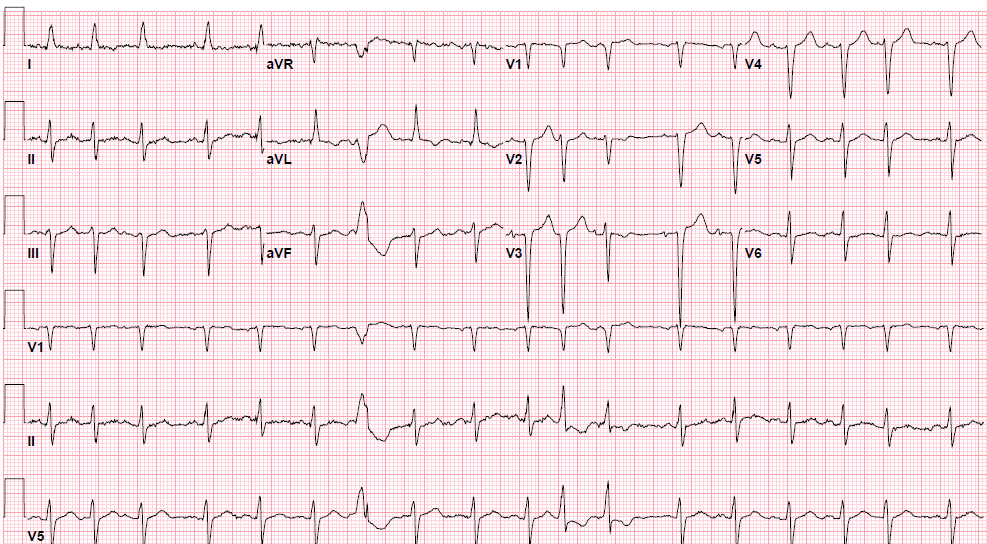
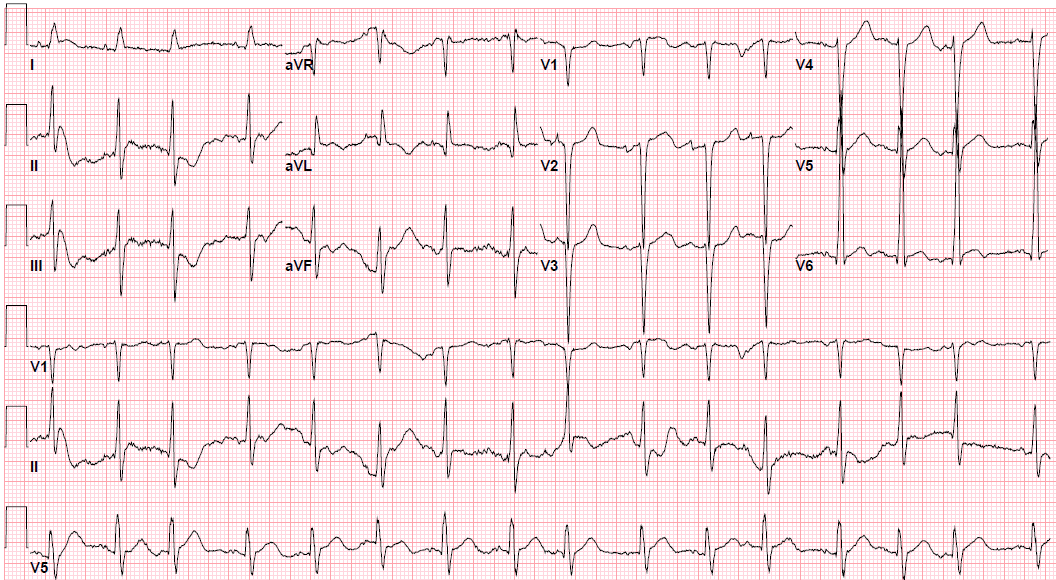
Pre-procedure EKG Heading
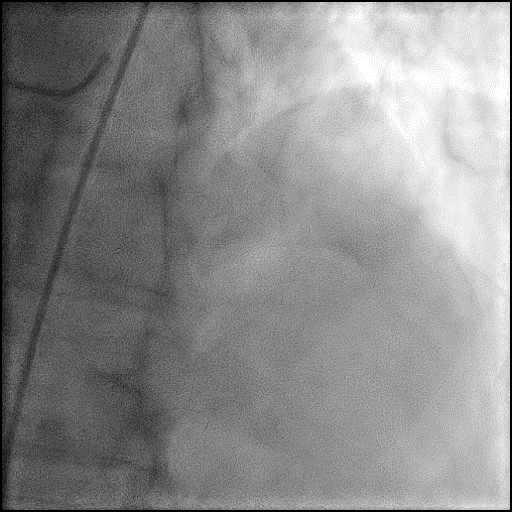
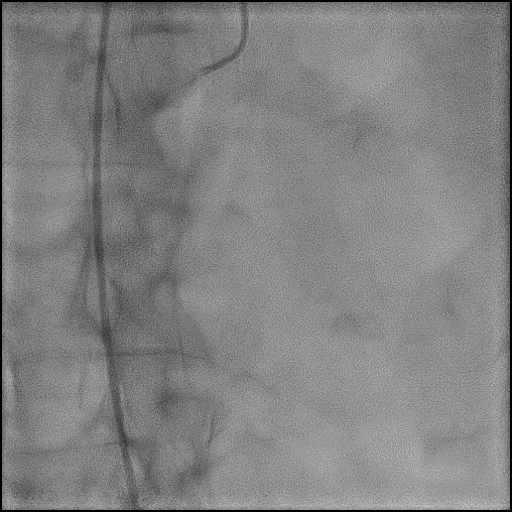
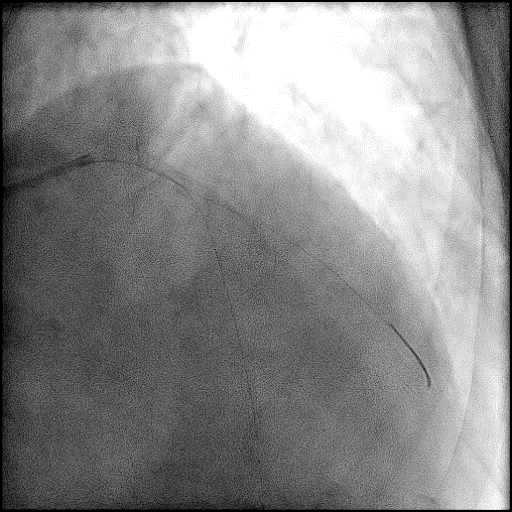
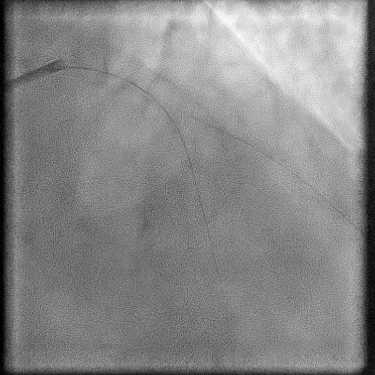
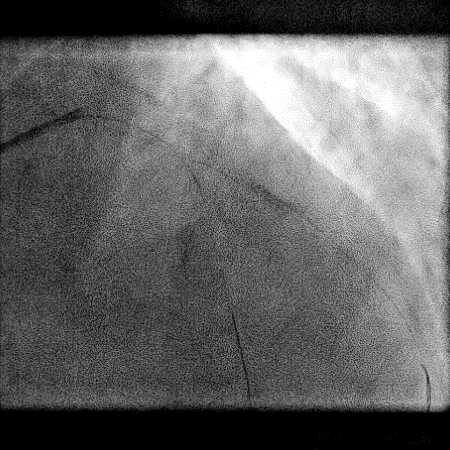
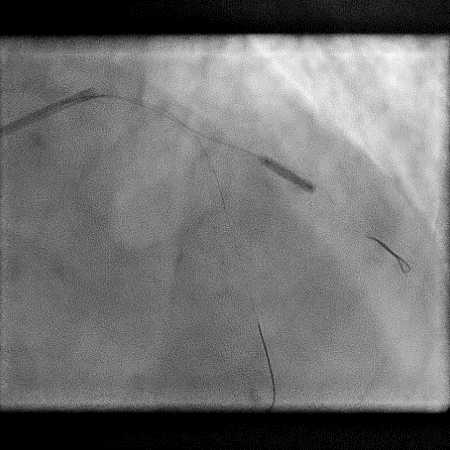
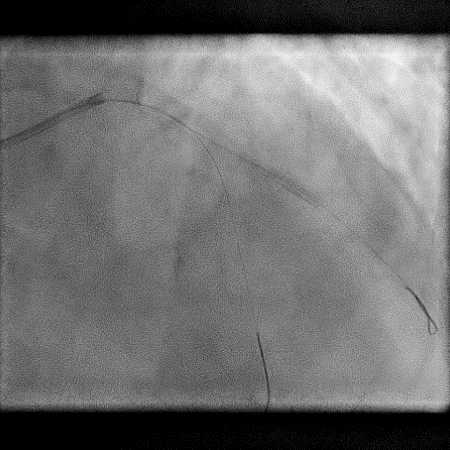
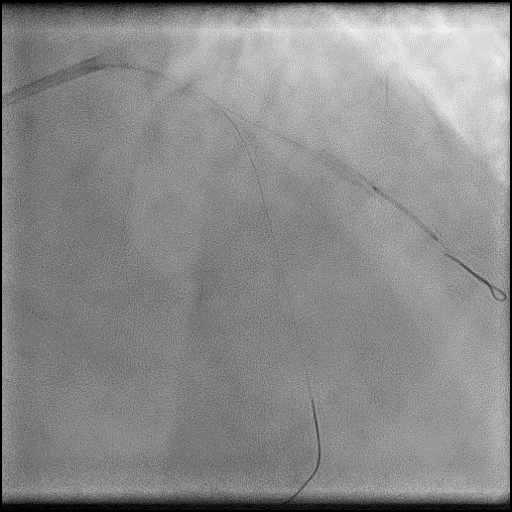
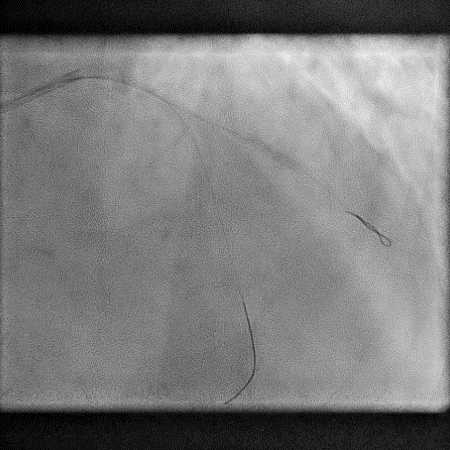
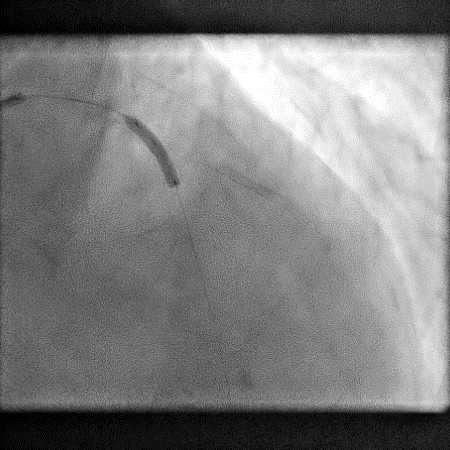
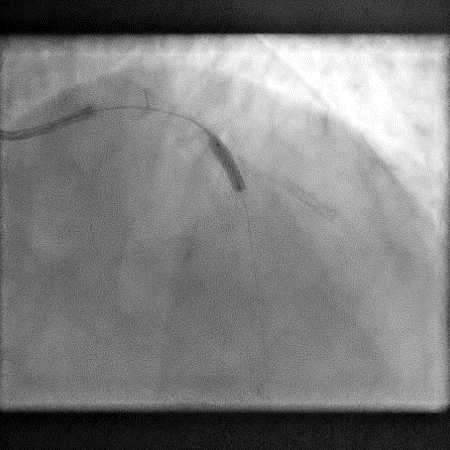
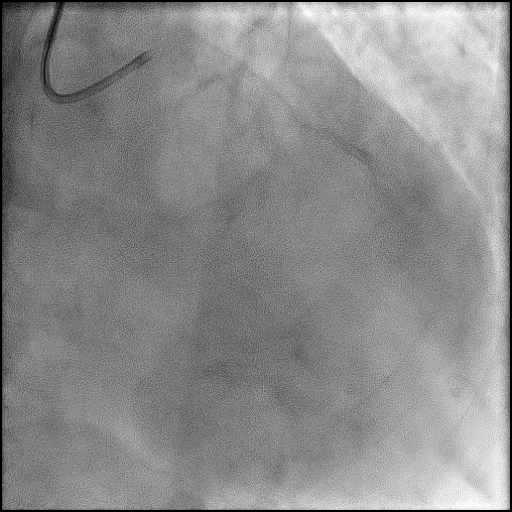
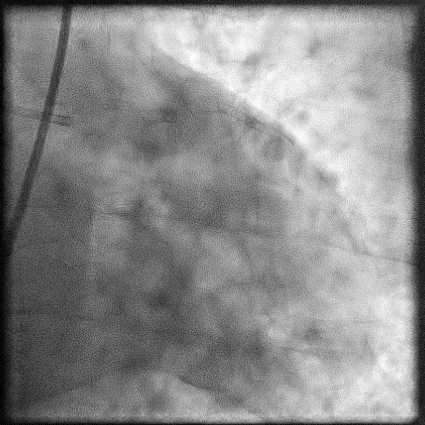
Angiograms
Post-procedure EKG
Post-procedure Echocardiography
Case Overview
- Underwent complex intervention of the LAD-D2 bifurcation.
- Pre-dilatation of the lesion in D2 resulted in a type 2 perforation.
- Perforation was inadequately sealed with prolonged balloon tamponade of the vessel.
- A covered stent was deployed with procedure being further complicated by a distal stent edge dissection. This was successfully treated with placement of another stent followed by post-dilatation of the stent.
- Procedure was continued and patient underwent successful LAD-D2 bifurcation stenting.
- Echocardiography showed presence of a small pericardial effusion without tamponade physiology.
- Troponin-I peaked at 12.07 ng/mL and CK-MB peaked at 67.8 ng/mL.
- Patient was discharged within 72 hours in stable condition.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- High pressure (pre)dilatation of a calcified vessel.
- How could the complication have been prevented?
- Lesions preparation with atherectomy with cutting balloon angioplasty prior to performing lesion pre-dilatation could have helped reduce calcified plaque burden, and made it safer to prep the vessel and reduce the chance of complications.
- Avoid aggressive, high pressure, over sized balloon dilatation of a calcified lesion as this can result in complications.
- Is there an alternate strategy that could have been used to manage the complication?
- Ellis Type 1 and 2 perforations usually seal spontaneously and are conservatively managed. Such patients should be closely monitored in the catheterization lab, and serial echocardiography should be performed, particularly if there is an Ellis Type 2 coronary perforation because it may lead to cardiac tamponade. Ellis Type 3 perforations are associated with increased risk of cardiac tamponade and mortality, and require immediate intervention/treatment. Ellis Type 3 Cavity Spilling perforation management is unclear. Usually they are conservatively managed, unless there is significant extravasation or the patient is symptomatic.
- Coronary perforation management algorithm:
- 1st: Prolonged balloon inflation: Position the balloon (or stent-balloon post stent deployment) just proximal or at the level of the perforation to prevent ongoing extravasation and development of hemo-pericardium. Ideally, the balloon to artery ratio should be 1:1. Inflate for 5-10 minutes followed by test deflations with contrast given in between inflations to evaluate the status of the perforation. If there is ongoing extravasation, re-inflate the balloon to stop further extravasation of blood into the pericardial space. This strategy helps stabilize the patients and gain control of the situation, while the operator prepares for echocardiography, pericardiocentesis, and more definitive treatment to seal the perforation.
- 2nd: Anticoagulation management: ‘STOP’ all anticoagulation immediately if you suspect or visualize a perforation. We consider ‘REVERSING’ heparin with protamine sulfate (to achieve ACT <225s) after coronary equipment is removed to prevent thrombosis within the vessel. If using bivalirudin, it can take up to 1-2 hours for its anticoagulation effect to a normalize after it is stopped. If patient was on glycoprotein IIB/IIIA inhibitors: For abciximab, consider giving platelet transfusion; tirofiban and eptifibatide have a short half life and their reversal can typically be achieved by stopping there infusion or in extreme cases with hemodialysis. Cangrelor has a short half life and its reversal can be achieved by stopping its infusion.
- 3rd: Covered stent: Standard of care for a perforation located in the proximal to mid segment of a vessel of appropriate size (≥2.5 mm), with no major side branch across the region where the stent will be placed. If a covered stent can be delivered to a distal vessel perforation, and the vessel is of appropriate size, covered stent placement to seal the perforation is reasonable. If the clinical situation allows, proceed with direct stent placement whenever possible using a single catheter or two-catheter (Ping-Pong) strategy. The stent should be quickly positioned and immediately deployed to high pressure. This should be followed by high pressure post-dilatation (18-20 atm) to achieve appropriate stent apposition.
- 4th: Embolization of distal vessel perforations: Non-surgical techniques for distal vessel embolization include: Coils, Gel Foams, Glues, Microspheres, Thrombin injection, Subcutaneous tissue, Autologous Blood Clots and multiple other agents (depending on what is available in an individual catheterization lab). Embolization leads to loss of vessel flow beyond point where embolized material is delivered and subsequent infarct in the vessel territory.
- 5th: Surgery Intervention: Ligation or suturing of the vessel for hemostasis with bypass grafting to the distal vessel. Pericardial patch/Teflon with possible bypass grafting to the distal vessel (consider this approach if vessel has multiple stents and/or presence of a subepicardial hematoma).
- What are the important learning points?
- Techniques associated with perforations: use of hydrophilic/extra stiff wires, atherectomy devices, cutting balloons, increase balloon-to-artery ratio, high pressure post-dilatation of stent.
- Angiographic findings associated with perforations: CTO, severe calcification, type C lesions, eccentric lesions, RCA or LCx lesion, tortuous vessels.
- When a complication occurs, an operator needs to quickly recognize and assess the situation to see if treatment of the complication should take precedence.
- In this case, intervention was deferred, anticoagulation stopped, and the perforation/dissection was treated immediately. The procedure was continued and successful intervention was performed. Failure to treat the perforation before continuing with the planned intervention could have lead to the development of cardiac tamponade.
- One of the management strategies in treating coronary perforation involves stopping anticoagulation therapy. If a stent is placed prior to treatment of a perforation, the reversal of anticoagulation could lead to a catastrophic event such as acute stent thrombosis.
- An operator must always remain vigilant for the presence of multiple complications. In this case, treatment of the perforation with a covered stent resulted in a Type C distal stent edge dissection which required additional treatment with a drug eluting stent.
- Type 1 perforation is similar to a type C dissection, and often difficult to distinguish from one another. Nonetheless, both can be managed with placement of a stent.