Perforation Type 3 Device – Case 3
Clinical Presentation
- 81-year-old female who presented to an outside hospital with a NSTEMI and cardiogenic shock. Cardiac catheterization showed severe 3-vessel CAD with LM involvement, severely reduced LV systolic function and moderate MR. An IABP was placed and she was transferred to another facility for emergent CABG, but deemed to high risk for surgical revascularization. Subsequently, she was transferred to our facility for emergent PCI.
Past Medical History
- HTN, HLD
- LVEF 25%
Clinical Variables
- Prior Cardiac Catheterization: LM 90% stenosis, proximal LAD 90% stenosis, proximal LCx subtotal, mid to distal RCA CTO.
Medications Heading
- Home Medications: Simvastatin, Alendronate, Calcium Carbonate-Vitamin D3, Enalapril, Mirtazapine
- Adjunct Pharmacotherapy: Clopidogrel, Heparin IV
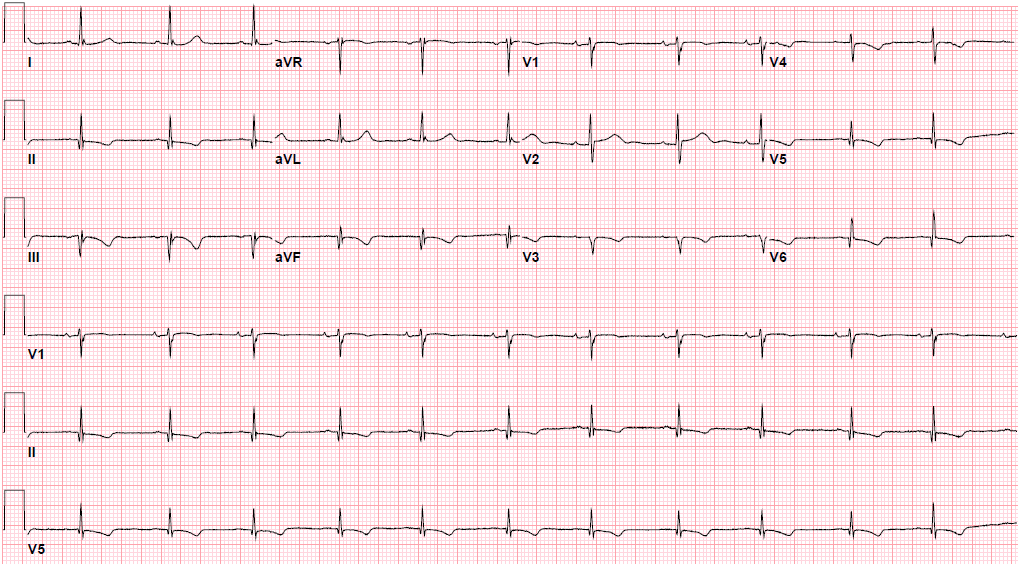
Pre-procedure EKG Heading
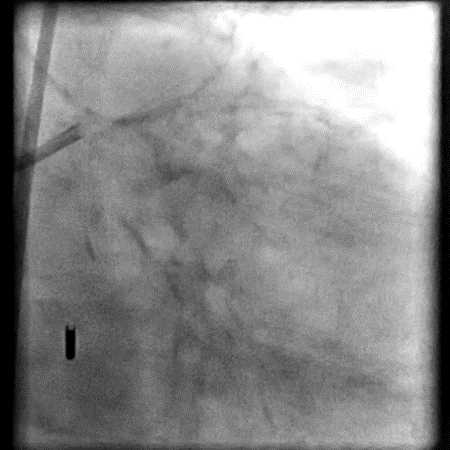
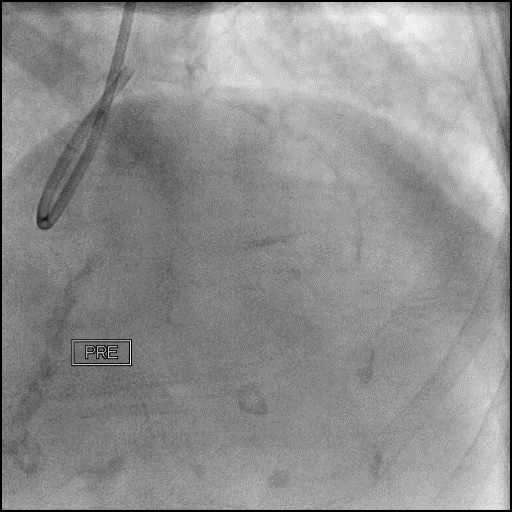
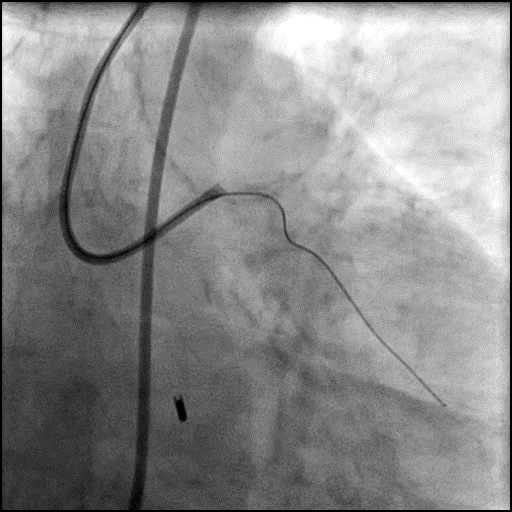
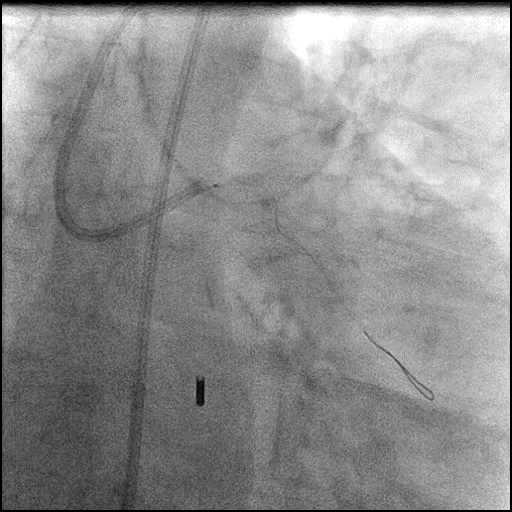
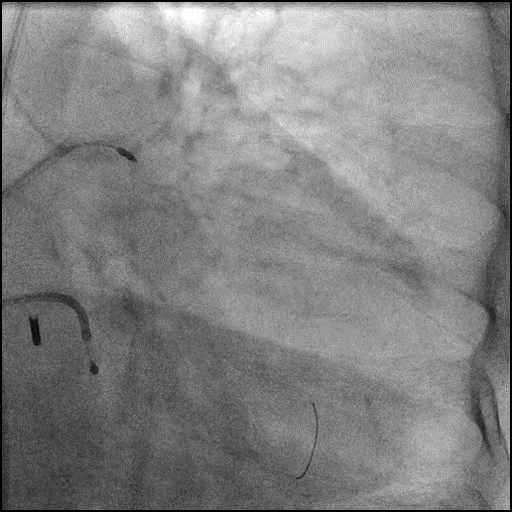
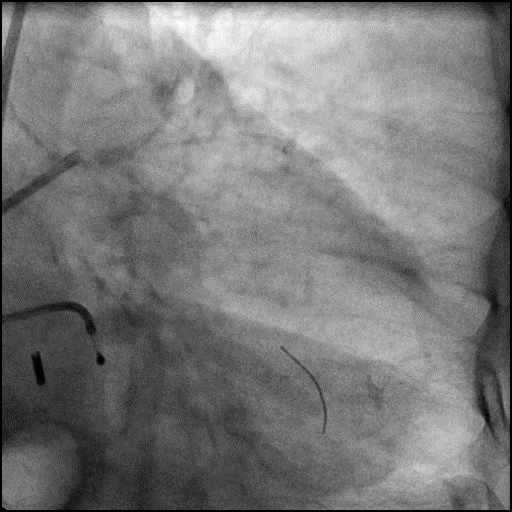
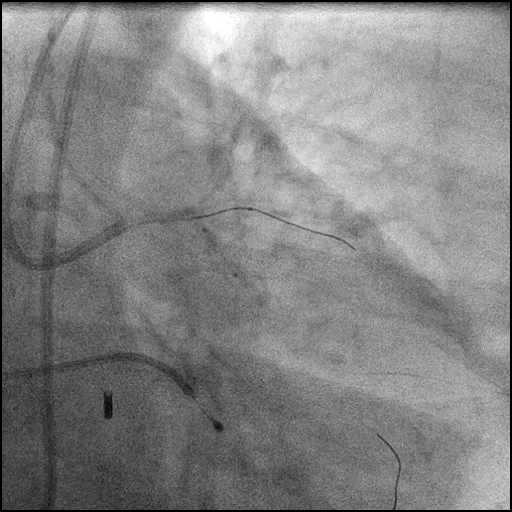
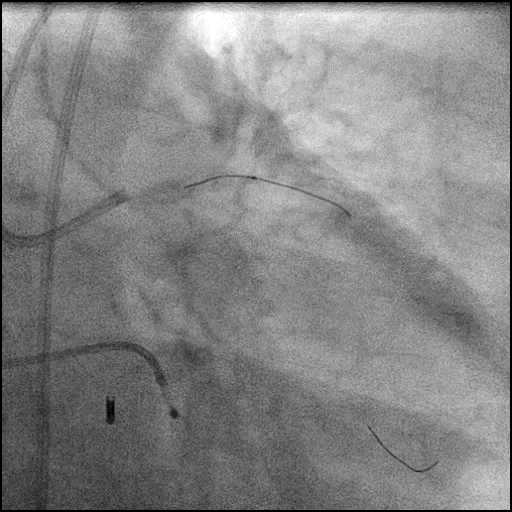
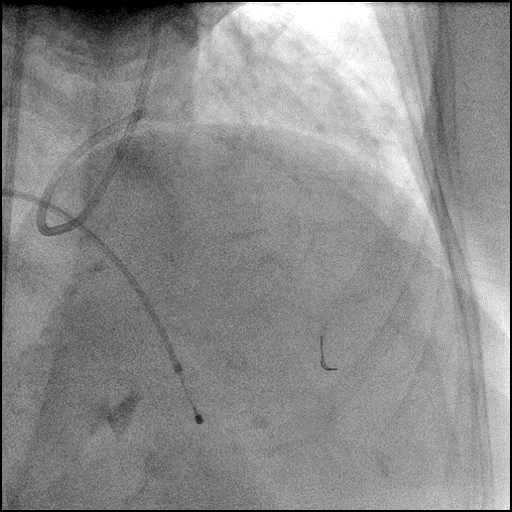
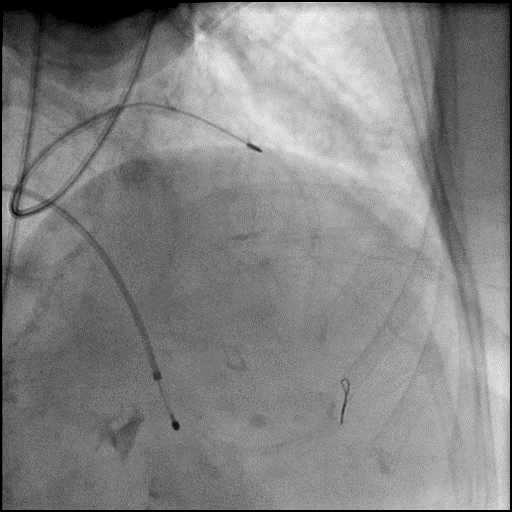
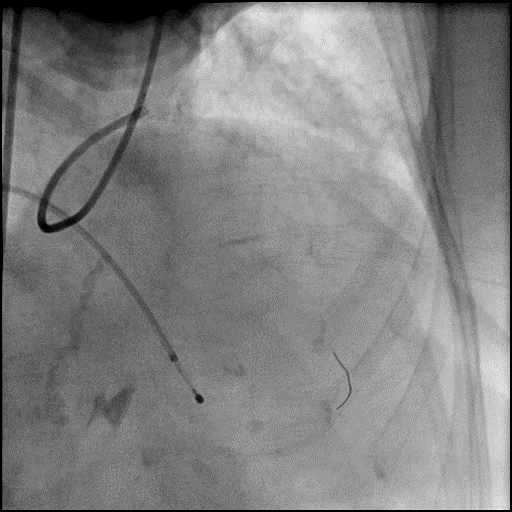
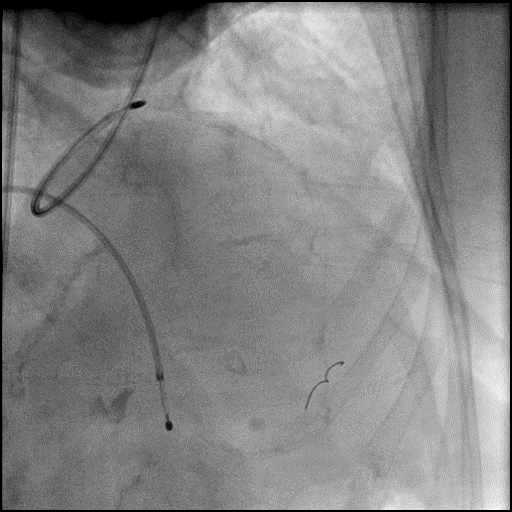
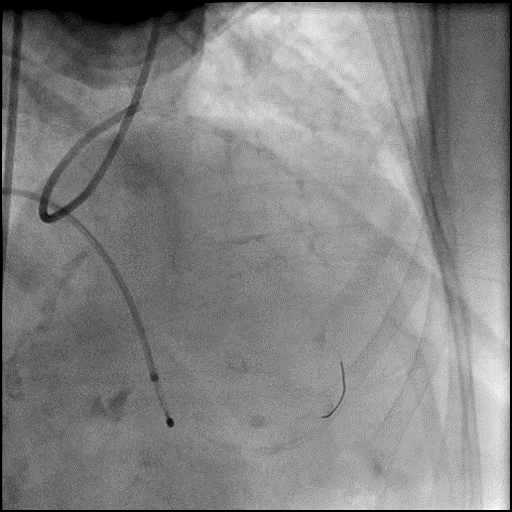
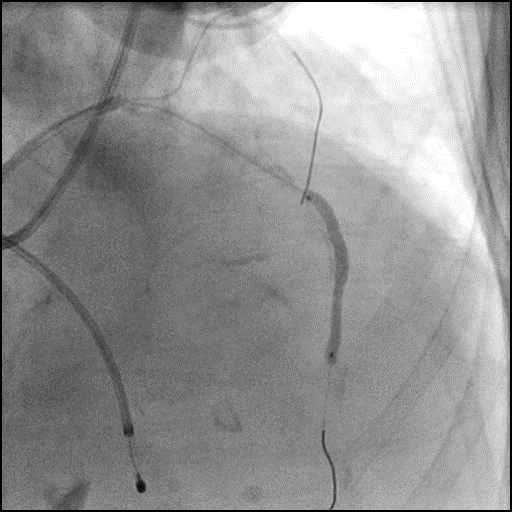
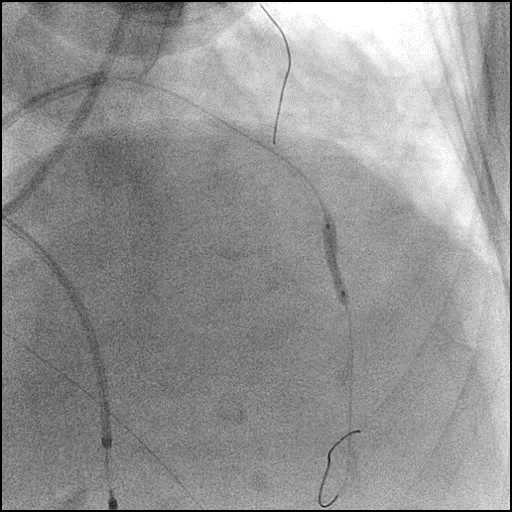
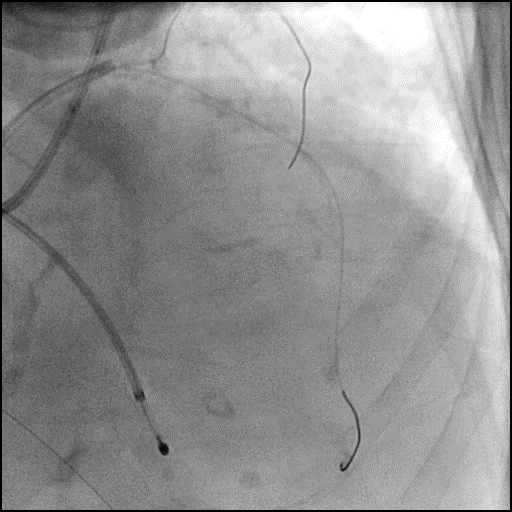
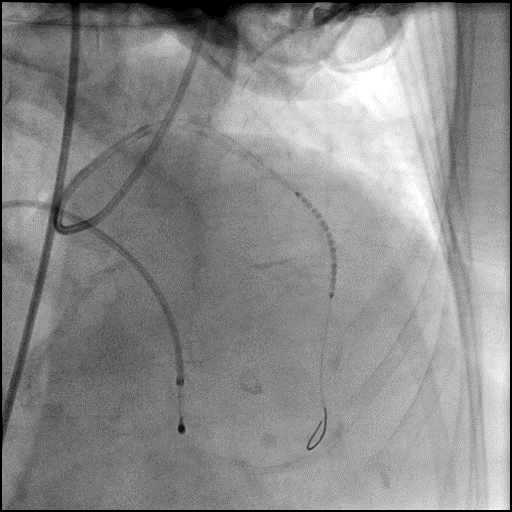
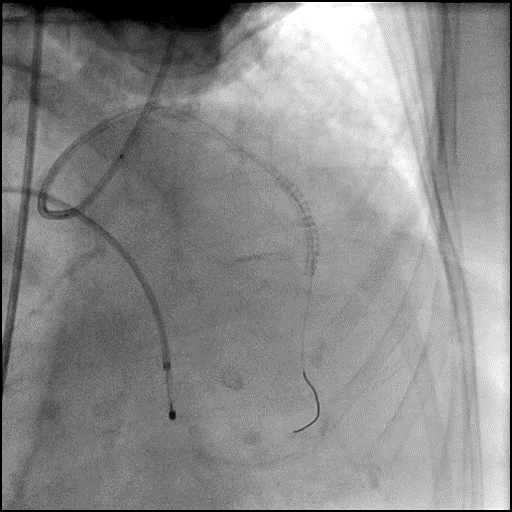
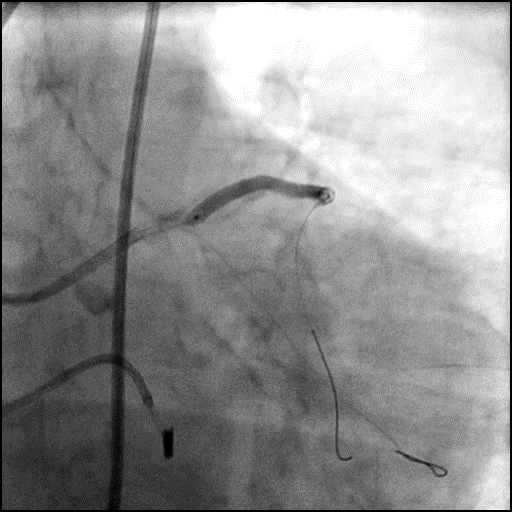
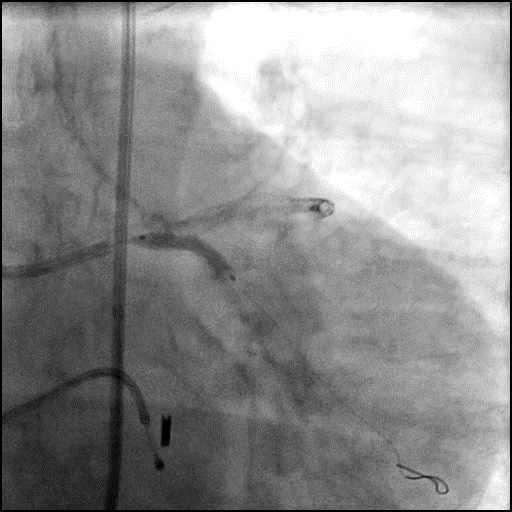
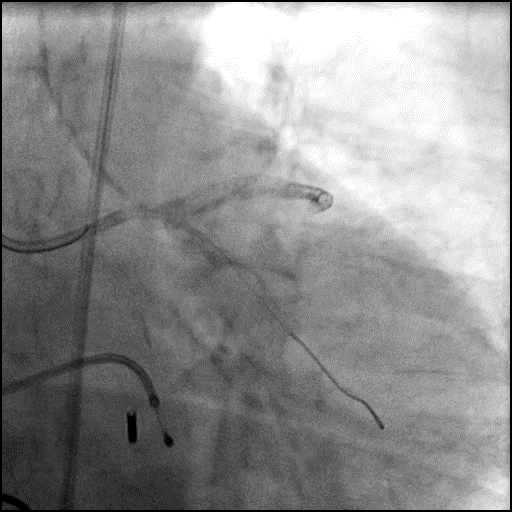
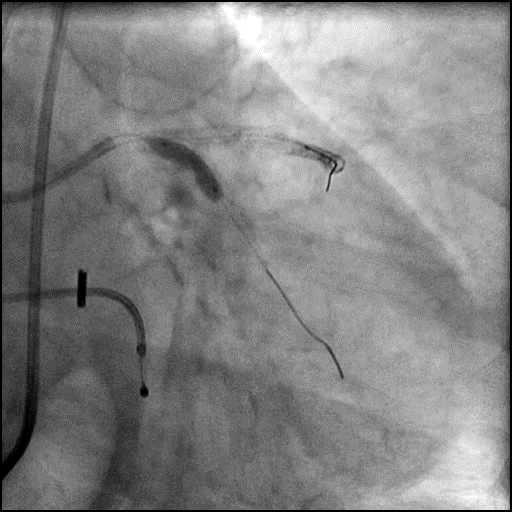
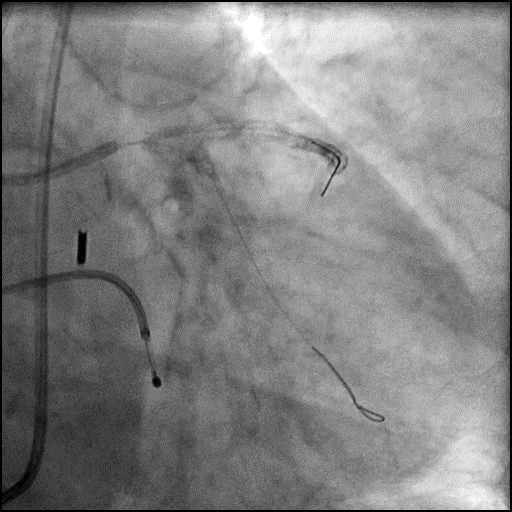
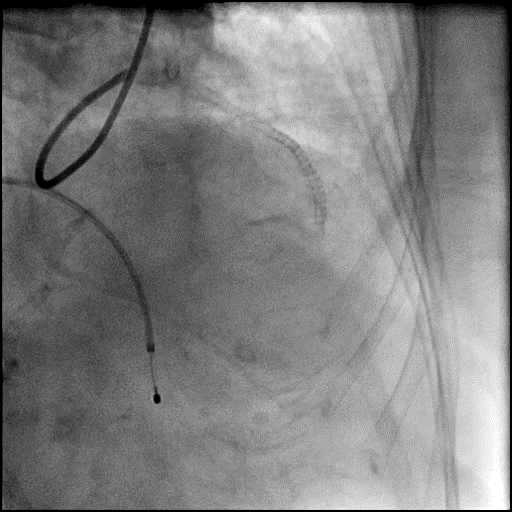
Angiograms
Post-procedure EKG
Case Overview
- Underwent intervention of the LM bifurcation.
- Rotational atherectomy was performed of the mid LAD. However, procedure was complicated by a type D dissection of the mid LAD.
- The LAD lesion/dissection was treated with high pressure balloon inflation, resulting in a type 3 LAD perforation.
- Perforation was inadequately sealed with prolonged balloon tamponade of the vessel, and a covered stent was placed sealing the perforation.
- Procedure was continued, and LM bifurcation stenting was performed using a T-Stent technique.
- Echocardiography showed presence of a small pericardial effusion without tamponade physiology.
- Troponin-I peaked at 30.1 ng/mL and CK-MB peaked at 57.0 ng/mL.
- Patient was discharged 10 days later without any sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- High pressure pre-dilatation of a calcified vessel.
- How could the complication have been prevented?
- Avoid aggressive, high pressure, over sized balloon dilatation of a calcified lesion as this can result in complications.
- Is there an alternate strategy that could have been used to manage the complication?
- Ellis Type 1 and 2 perforations usually seal spontaneously and are conservatively managed. Such patients should be closely monitored in the catheterization lab, and serial echocardiography should be performed, particularly if there is an Ellis Type 2 coronary perforation because it may lead to cardiac tamponade. Ellis Type 3 perforations are associated with increased risk of cardiac tamponade and mortality, and require immediate intervention/treatment. Ellis Type 3 Cavity Spilling perforation management is unclear. Usually they are conservatively managed, unless there is significant extravasation or the patient is symptomatic.
- Coronary perforation management algorithm:
- 1st: Prolonged balloon inflation: Position the balloon (or stent-balloon post stent deployment) just proximal or at the level of the perforation to prevent ongoing extravasation and development of hemo-pericardium. Ideally, the balloon to artery ratio should be 1:1. Inflate for 5-10 minutes followed by test deflations with contrast given in between inflations to evaluate the status of the perforation. If there is ongoing extravasation, re-inflate the balloon to stop further extravasation of blood into the pericardial space. This strategy helps stabilize the patients and gain control of the situation, while the operator prepares for echocardiography, pericardiocentesis, and more definitive treatment to seal the perforation.
- 2nd: Anticoagulation management: ‘STOP’ all anticoagulation immediately if you suspect or visualize a perforation. We consider ‘REVERSING’ heparin with protamine sulfate (to achieve ACT <225s) after coronary equipment is removed to prevent thrombosis within the vessel. If using bivalirudin, it can take up to 1-2 hours for its anticoagulation effect to a normalize after it is stopped. If patient was on glycoprotein IIB/IIIA inhibitors: For abciximab, consider giving platelet transfusion; tirofiban and eptifibatide have a short half life and their reversal can typically be achieved by stopping there infusion or in extreme cases with hemodialysis. Cangrelor has a short half life and its reversal can be achieved by stopping its infusion.
- 3rd: Covered stent: Standard of care for a perforation located in the proximal to mid segment of a vessel of appropriate size (≥2.5 mm), with no major side branch across the region where the stent will be placed. If a covered stent can be delivered to a distal vessel perforation, and the vessel is of appropriate size, covered stent placement to seal the perforation is reasonable. If the clinical situation allows, proceed with direct stent placement whenever possible using a single catheter or two-catheter (Ping-Pong) strategy. The stent should be quickly positioned and immediately deployed to high pressure. This should be followed by high pressure post-dilatation (18-20 atm) to achieve appropriate stent apposition.
- 4th: Embolization of distal vessel perforations: Non-surgical techniques for distal vessel embolization include: Coils, Gel Foams, Glues, Microspheres, Thrombin injection, Subcutaneous tissue, Autologous Blood Clots and multiple other agents (depending on what is available in an individual catheterization lab). Embolization leads to loss of vessel flow beyond point where embolized material is delivered and subsequent infarct in the vessel territory.
- 5th: Surgery Intervention: Ligation or suturing of the vessel for hemostasis with bypass grafting to the distal vessel. Pericardial patch/Teflon with possible bypass grafting to the distal vessel (consider this approach if vessel has multiple stents and/or presence of a subepicardial hematoma).
- What are the important learning points?
- Device related perforations tend to be more catastrophic than wire related perforations. This is because devices cause trauma and disrupt the integrity of the vessel wall.
- After any device is inflated inside a coronary vessel, the device should be retracted into the guide and a gentle contrast injection should be performed. If there is a perforation, the device (balloon) should be immediately positioned in the correct location (just proximal to the perforation) and inflated to tamponade the vessel. This strategy gives an operator time to obtain equipment to perform more definitive therapy (i.e. covered stent, coils, embolization materials etc.) and prevent hemodynamic decompensation from cardiac tamponade.
- When there is a type 3 perforation, a stat echocardiogram needs to be performed, consideration needs to be given to stop/reverse anticoagulation, and to perform a pericardiocentesis. If immediately available, quickly deliver a balloon just proximal or at the level of the perforation and inflate it to tamponade the vessel (gives you time to plan for more definitive therapy). In the presence of hemodynamic instability or large perforation, you should consider using the Ping-Pong technique to deliver a covered stent.
- If it is difficult to localize the point of extravasation or if there are multiple extravasation jets, we recommend using a longer stent to cover the perforation.
- Delivery of a covered stent:
- A covered stent can be delivered using the same guide catheter after removal and retrieval of the balloon, if there is no significant hemodynamic decompromise and in the absence of a large perforation present. If using this strategy, an operator needs to act quickly because once the balloon is deflated, there will be ongoing coronary extravasation into the pericardial space.
- Alternatively, a second guide catheter strategy can be used for delivering a covered stent. To do this, obtain alternate access, advance a second guide catheter, disengage the first guide catheter and intubate the perforated artery with the new guide catheter (PING-PONG technique). Next, advance a second guidewire to the proximal edge of the inflated balloon, deflate the balloon, advance the wire to the distal vessel and then immediately re-inflate the balloon. The covered stent is advanced over the second guidewire until proximal to the inflated balloon. Then deflate the balloon and remove it along with the first guidewire (into the initial guide catheter), and quickly position the covered stent and immediately deploy it to high pressure. This should be followed by high pressure post-dilatation (18-20 atm) to achieve appropriate stent apposition.