Wire Bias – Case 1
Clinical Presentation
- 71-year-old male who presented with chest pain (CCS Class II) and was referred for staged PCI of the LCx-LPL1.
Past Medical History
- HTN, HLD, Former Tobacco Use, CAD s/p PCI, PAD, Rheumatoid Arthritis
- LVEF 62%
Clinical Variables
- Prior Cardiac Catheterization: OM1 50-60% stenosis, LPL1 80-90% stenosis, proximal RCA subtotal, mid RCA 80-90% stenosis. S/p successful PCI of the RCA.
Medications
- Home Medications: Aspirin, Clopidogrel, Atorvastatin, Metoprolol Succinate, Amlodipine, Isosorbide Mononitrate
- Adjunct Pharmacotherapy: Clopidogrel, Bivalirudin
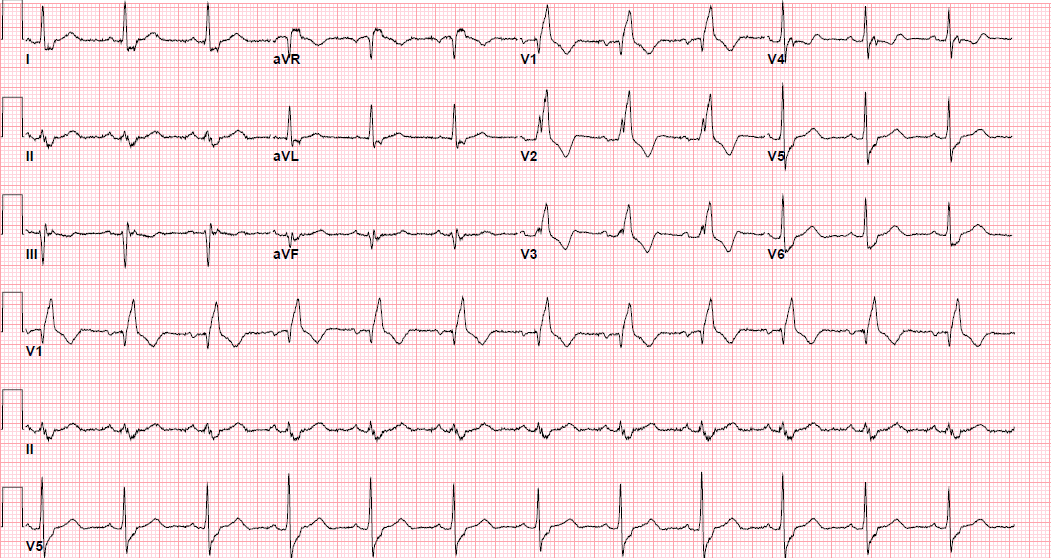
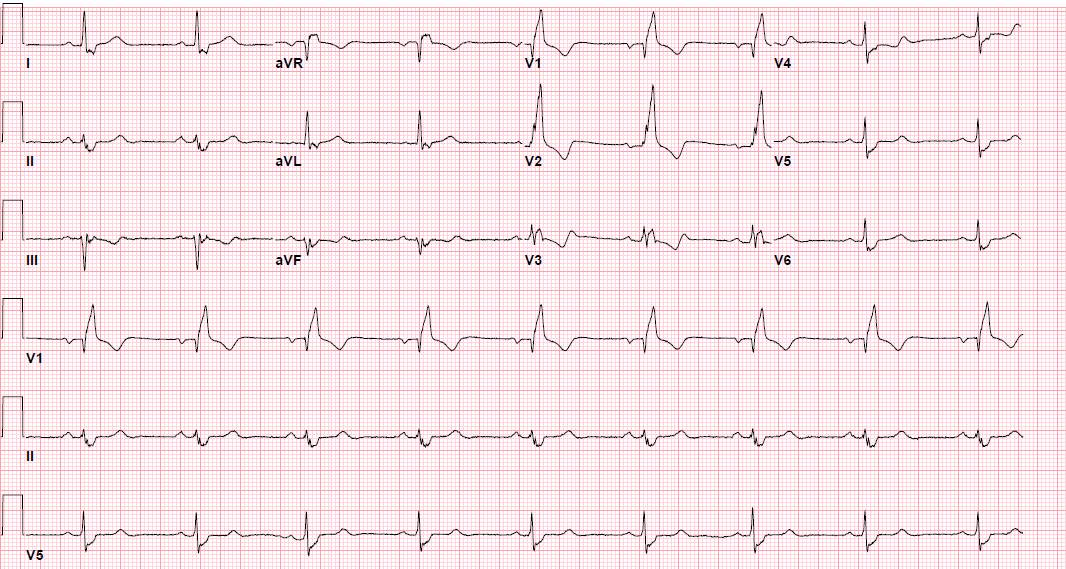
Pre-procedure EKG
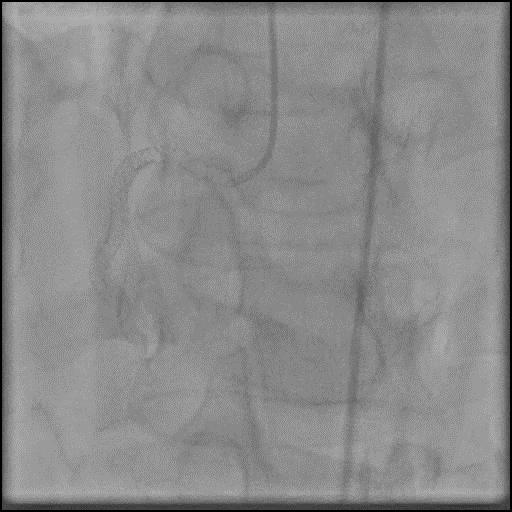
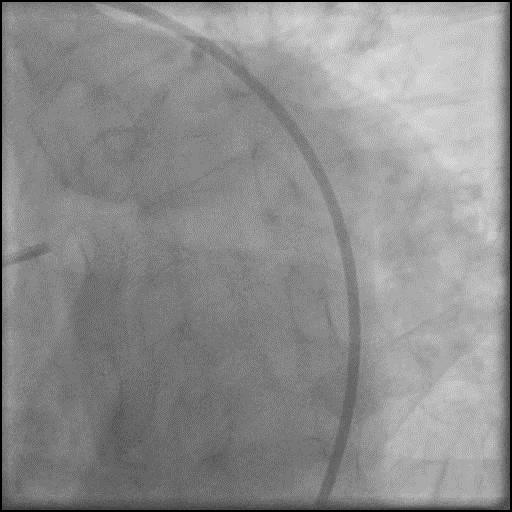
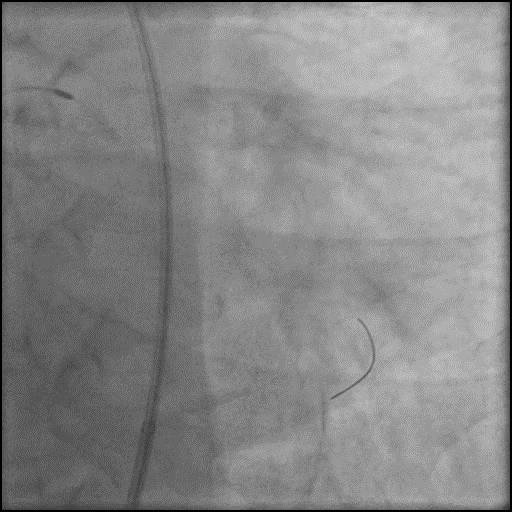
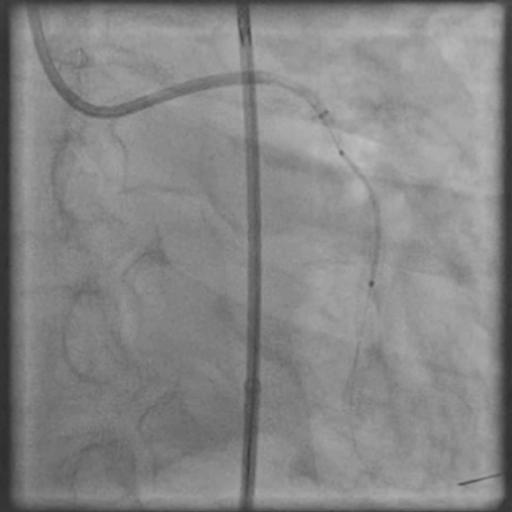
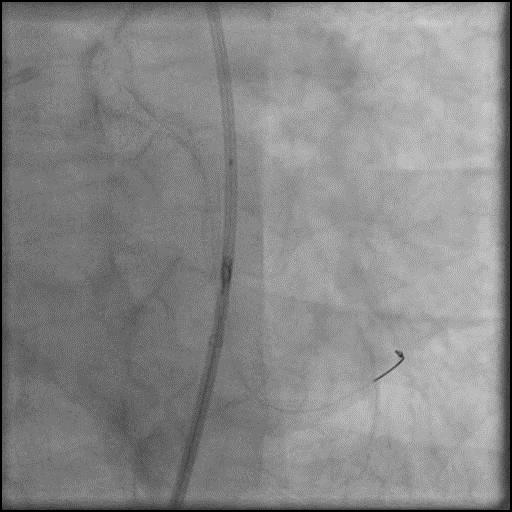
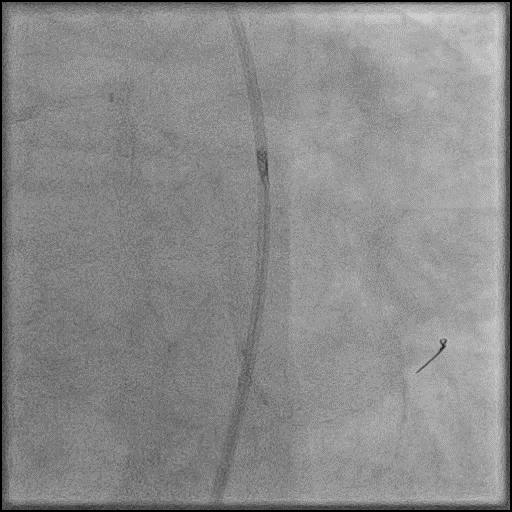
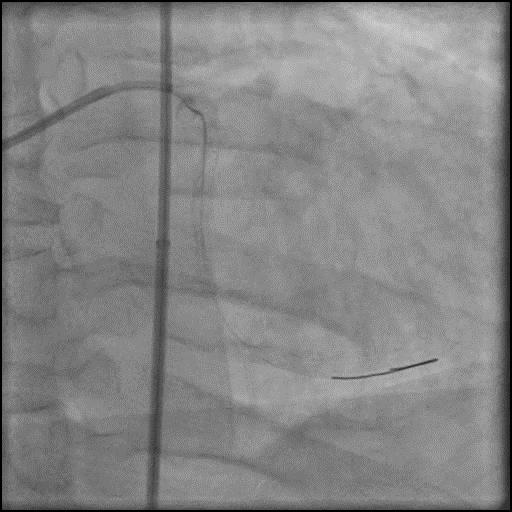
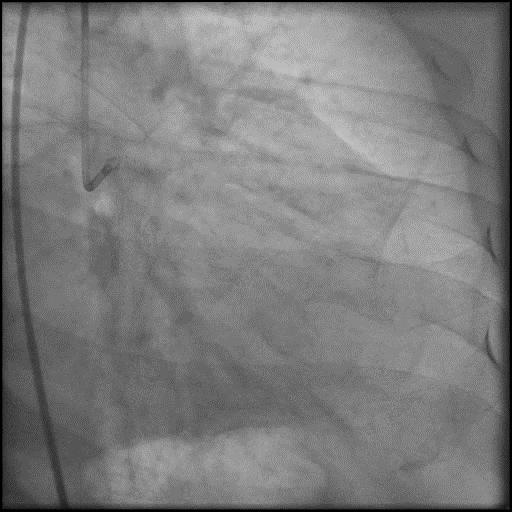
Angiograms
Post-procedure EKG
Case Overview
- Underwent intervention of the LCx.
- Procedure was complicated by a type D dissection following rotational atherectomy due to severe wire bias.
- Dissection was successfully treated with placement of two overlapping stents.
- Troponin-I peaked at 0.56 ng/mL and CK-MB peaked at 6.6 ng/mL.
- Patient was discharged home the next day without further sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- Wire bias altering the trajectory of the rotational atherectomy device.
- How could the complication have been prevented?
- Use of a guide catheter which provides coaxial engagement helps reduce unfavorable guidewire bias.
- Pay close attention to the ACT during the procedure and dose anticoagulation accordingly to maintain ACT >300 prior to performing an intervention (Hemochron machine).
- Avoid performing rotational or orbital atherectomy in vessels which are highly tortuous with severe wire bias. As an alternative, consider performing laser atherectomy, intravascular Lithotripsy (IVL) (off label use in the USA), cutting balloon angioplasty for plaque modification and/or treatment of calcified CAD.
- Is there an alternate strategy that could have been used to manage the complication?
- Wire bias could have possibly been prevented by use of a Rota Extra Support wire instead of a Rota Floppy wire.
- A stiffer wire can sometimes be used to straighten the vessel or lesion to lessen the resistance and possibly reduce wire bias.
- What are the important learning points?
- This is a Type D dissection because of the presence of a spiral filling defect which is clearly outlined with persistence of extraluminal contrast which is present after contrast injection.
- Need to be extremely cautious when using atherectomy devices in extremely tortuous vessels. Wire bias is more likely to occur in tortuous vessels and it increasing the risk of dissection or perforation.
- Guidewire bias is when you have divergence from the central axis of the vessel and can result in ablation of normal tissue if the tension on the wall exceeds the elasticity of the vessel. To reduce sidewall tension:
- Keep the tip of the guidewire just beyond the lesion
- Use a stepped-burr approach starting with an undersized burr is preferred
- When advancing the burr, do so at low speed to reduce there is less tension in the wire
- There are two kinds wires that can be used when performing rotational atherectomy:
- Rota Floppy Wire: Used in most cases, and is more flexible with a longer taper and shorter spring tip compared with the Rota Extra Support wire. This wire causes less vessel straightening, less wire bias, and allows for atherectomy along the greater curvature of an angulated lesion.
- Rota Extra Support Wire: Used in tortuous vessels or in situations when one suspects wire bias as a reason for the burr not crossing the lesion. Wire is stiffer, and with a shorter taper and longer spring tip compared with the Rota floppy wire. This wire causes the vessel to straighten out the tortuosity to allow the burr to advance to a lesion, and is useful in ablation of plaque at the lesser curvature of angulated lesions, aorto-ostial lesions and distal lesions. However, this wire can result in proximal vessel spasm and ‘pseudo-stenosis’.
- A stiffer guidewire does not always result in unfavorable bias, and may result in a favorable bias, particularly when performing rotabalation of an angulated and heavily calcified lesion.
- Once a dissection is identified, rotational atherectomy should be stopped, and primary focus should be on maintaining wire position. After confirming the wire is in the true lumen, the dissection should be treated accordingly with balloon inflation and placement of a stent.