Perforation – Atherectomy – Case 1
Clinical Presentation
- 54-year-old female who presented with chest pain (CCS Class III) and was referred for IVBT.
Past Medical History
- HTN, HLD, Active Tobacco Use, CAD s/p Multiple PCI’s, Sarcoidosis, Neuropathy (receiving IVIG therapy), Pulmonary Fibrosis, COPD
- LVEF 70%
Clinical Variables
- Stress MPI: Moderate ischemia along the antero-lateral wall.
Medications
- Home Medications: Aspirin, Ticagrelor, Atorvastatin, Metoprolol Succinate, Nifedipine ER, Hydrocholorothiazide, Mycophenolate Mofetil, Mirtazapine, Fluticasone-Salmeterol, Aclidinium Bromide, Colchicine, Cylcobenzaprine, Famotidine
- Adjunct Pharmacotherapy: Ticagrelor, Bivalirudin
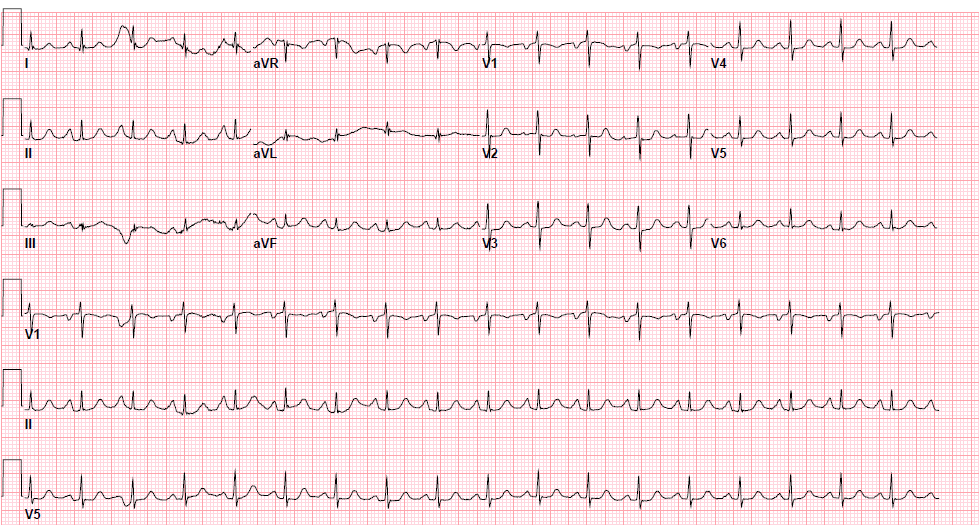
Pre-procedure EKG
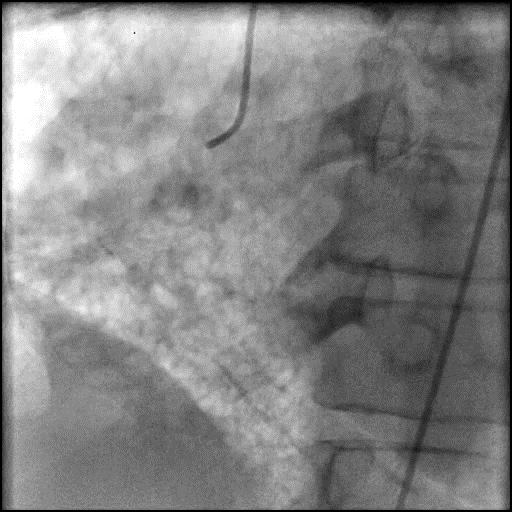
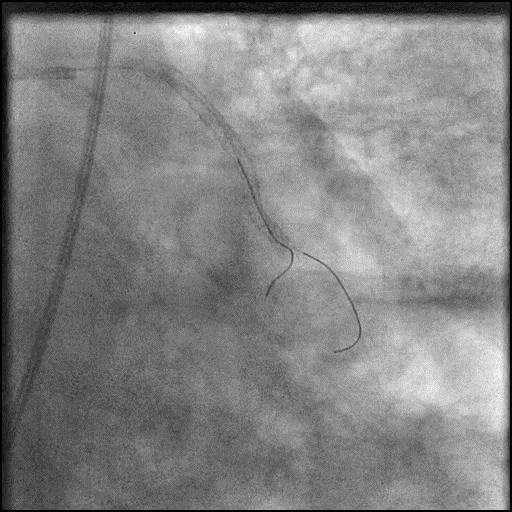
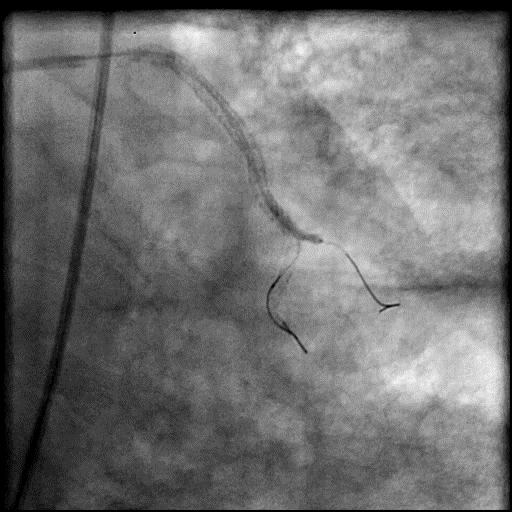
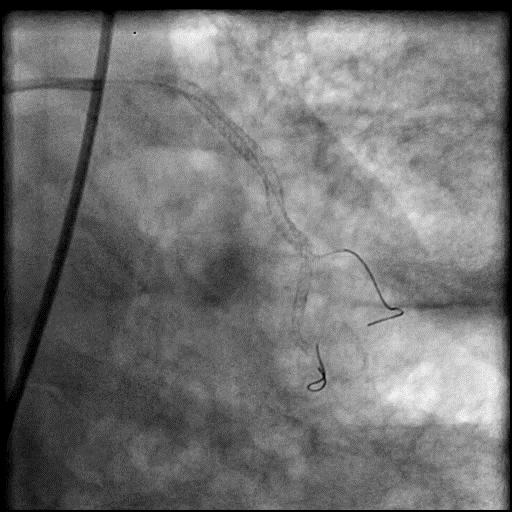
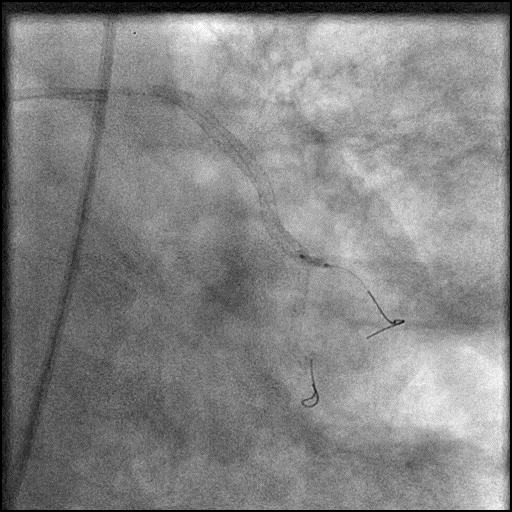
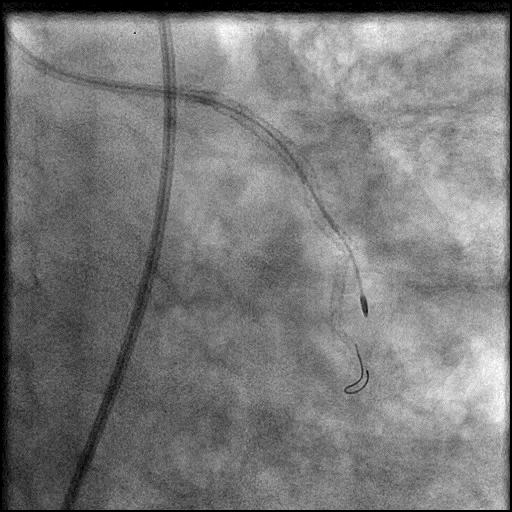
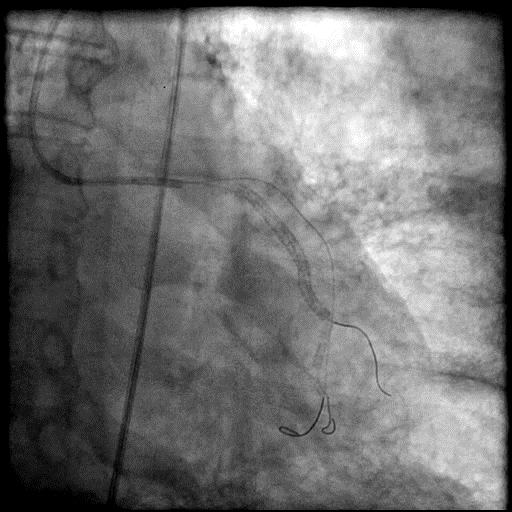
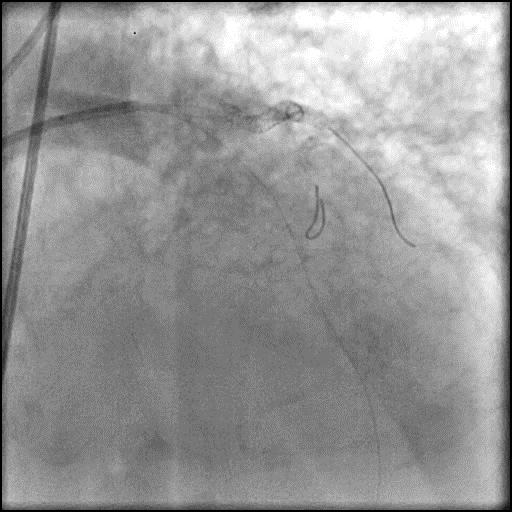
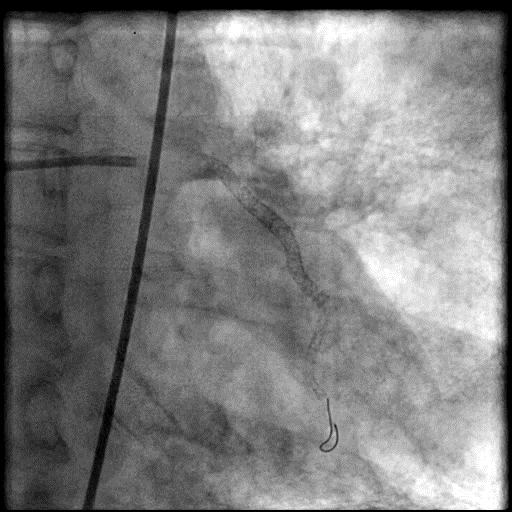
Angiograms
Post-procedure EKG
Post-procedure Echocardiography
Case Overview
- Underwent intervention of D1.
- During rotational atherectomy in a tortuous lesion, the burr transected the rota wire and the burr advanced outside the lumen, causing a type 3 perforation.
- Antiplatelet/Anticoagulation therapy was immediately stopped, and prolonged balloon inflation to tamponade the vessel was performed.
- Thrombus formed leading to abrupt closure of D1, sealing the perforation.
- Procedure was further complicated by AVC due to embolization of thrombus into the LAD.
- Aspiration thrombectomy of the LAD was performed, successfully restoring flow in the LAD.
- Echocardiography showed no pericardial effusion or tamponade physiology.
- Troponin-I peaked at 5.71 ng/mL and CK-MB peaked at 28.7 ng/mL.
- Patient was discharged home 2 days later without further sequelae.
Learning Objectives
- What is the likely explanation or reason why the complication occurred?
- While performing stent ablation using rotational atherectomy of an angulated, long segment in-stent restenosis lesion with multiple stent layers, the stiffness of the rota wire created significant wire bias. Although, RA was performed cautiously, it resulted in wire fracture and subsequent burr advancement outside the vessel leading to a type 3 coronary perforation.
- In this case, stopping anticoagulation and prolonged balloon inflation to tamponade the perforated vessel (D1) resulted in AVC from thrombus formation with thrombus then embolizating to the LAD.
- How could the complication have been prevented?
- Rotational atherectomy should be considered on a case by case bases, especially when the lesion is long, severely calcified and/or with severe angulation/tortuosity. In this case we should have been less aggressive with performing rotational atherectomy and considered performing cutting/scoring balloon angioplasty in the distal RCA/RPDA.
- Technical modifications to prevent rotablation related perforation:
- Use a small burr size (start with 1.25 burr)
- Bending the wire technique
- Use of Rota Extra Support wire
- Pre-dilatation with small balloon
- Avoid GP IIB/IIA prior to rotablation
- Is there an alternate strategy that could have been used to manage the complication?
- Ellis Type 1 and 2 perforations usually seal spontaneously and are conservatively managed. Such patients should be closely monitored in the catheterization lab, and serial echocardiography should be performed, particularly if there is an Ellis Type 2 coronary perforation because it may lead to cardiac tamponade. Ellis Type 3 perforations are associated with increased risk of cardiac tamponade and mortality, and require immediate intervention/treatment. Ellis Type 3 Cavity Spilling perforation management is unclear. Usually they are conservatively managed, unless there is significant extravasation or the patient is symptomatic.
- Coronary perforation management algorithm:
- 1st: Prolonged balloon inflation: Position the balloon (or stent-balloon post stent deployment) just proximal or at the level of the perforation to prevent ongoing extravasation and development of hemo-pericardium. Ideally, the balloon to artery ratio should be 1:1. Inflate for 5-10 minutes followed by test deflations with contrast given in between inflations to evaluate the status of the perforation. If there is ongoing extravasation, re-inflate the balloon to stop further extravasation of blood into the pericardial space. This strategy helps stabilize the patients and gain control of the situation, while the operator prepares for echocardiography, pericardiocentesis, and more definitive treatment to seal the perforation.
- 2nd: Anticoagulation management: ‘STOP’ all anticoagulation immediately if you suspect or visualize a perforation. We consider ‘REVERSING’ heparin with protamine sulfate (to achieve ACT <225s) after coronary equipment is removed to prevent thrombosis within the vessel. If using bivalirudin, it can take up to 1-2 hours for its anticoagulation effect to a normalize after it is stopped. If patient was on glycoprotein IIB/IIIA inhibitors: For abciximab, consider giving platelet transfusion; tirofiban and eptifibatide have a short half life and their reversal can typically be achieved by stopping there infusion or in extreme cases with hemodialysis. Cangrelor has a short half life and its reversal can be achieved by stopping its infusion.
- 3rd: Covered stent: Standard of care for a perforation located in the proximal to mid segment of a vessel of appropriate size (≥2.5 mm), with no major side branch across the region where the stent will be placed. If a covered stent can be delivered to a distal vessel perforation, and the vessel is of appropriate size, covered stent placement to seal the perforation is reasonable. If the clinical situation allows, proceed with direct stent placement whenever possible using a single catheter or two-catheter (Ping-Pong) strategy. The stent should be quickly positioned and immediately deployed to high pressure. This should be followed by high pressure post-dilatation (18-20 atm) to achieve appropriate stent apposition.
- 4th: Embolization of distal vessel perforations: Non-surgical techniques for distal vessel embolization include: Coils, Gel Foams, Glues, Microspheres, Thrombin injection, Subcutaneous tissue, Autologous Blood Clots and multiple other agents (depending on what is available in an individual catheterization lab). Embolization leads to loss of vessel flow beyond point where embolized material is delivered and subsequent infarct in the vessel territory.
- 5th: Surgery Intervention: Ligation or suturing of the vessel for hemostasis with bypass grafting to the distal vessel. Pericardial patch/Teflon with possible bypass grafting to the distal vessel (consider this approach if vessel has multiple stents and/or presence of a subepicardial hematoma).
- What are the important learning points?
- Need to be extremely cautious when using atherectomy devices, especially in tortuous vessels where there is increased guidewire bias, which increases the risk of dissection and perforation.
- The harbinger of rotational atherectomy related complications is intermittent stalling of the burr. Feedback for from the rotablation system is important in preempting complications and can be perceived by the following:
- Visual: smooth advancement under fluoroscopy and monitoring decelerations in the speed (RPM) on the monitor
- Auditory: listen for changes in the frequency of sound as the burr encounters resistance
- Tactile: advancer knob resistance or driveshaft vibration (consider the following: excessive load on burr, too rapid advancement, a kini in the drive shaft coil, too large of a burr)
- Guidewire bias is when you have divergence from the central axis of the vessel and can result in ablation of normal tissue if the tension on the wall exceeds the elasticity of the vessel. To reduce sidewall tension:
- Keep the tip of the guidewire just beyond the lesion
- Use a stepped-burr approach starting with an undersized burr is preferred
- When advancing the burr, do so at low speed to reduce there is less tension in the wire
- There are two kinds wires that can be used when performing rotational atherectomy:
- Rota Floppy Wire: Used in most cases, and is more flexible with a longer taper and shorter spring tip compared with the Rota Extra Support wire. This wire causes less vessel straightening, less wire bias, and allows for atherectomy along the greater curvature of an angulated lesion.
- Rota Extra Support Wire: Used in tortuous vessels or in situations when one suspects wire bias as a reason for the burr not crossing the lesion. Wire is stiffer, and with a shorter taper and longer spring tip compared with the Rota floppy wire. This wire causes the vessel to straighten out the tortuosity to allow the burr to advance to a lesion, and is useful in ablation of plaque at the lesser curvature of angulated lesions, aorto-ostial lesions and distal lesions. However, this wire can result in proximal vessel spasm and ‘pseudo-stenosis’.
- A stiffer guidewire does not always result in unfavorable bias, and may result in a favorable bias, particularly when performing rotabalation of an angulated and heavily calcified lesion.
- When a wire is fractured within a coronary vessel, an attempt should be made to percutaneously retrieve the remnant wire or exclude it with a stent, particularly if the remnant wire is located proximal in a large caliber vessel. If the remnant wire is located more distal, it is reasonable to defer intervention. In this case, the fractured wire was located more distal within the vessel, and attempts to retrieve the remnant wire were abandoned as the vessel had already thrombosed due to prolonged balloon inflation to tamponade the vessel.