Mechanical Circulatory Support
With advances in technologies and PCI technique, interventional cardiologists are able to perform complex coronary intervention than before. Mechanical circulatory support devices (MCS) are designed to provide hemodynamic support for a wide range of clinical conditions, ranging from prophylactic insertion for high-risk invasive coronary artery procedures to the management of cardiogenic shock, acute decompensated heart failure, or cardiopulmonary arrest.
The two commonly used MCS during PCI for hemodynamic support are IABP and Impella systems (Abiomed).
Indication of hemodynamic support in PCI
- Cardiogenic shock
- Severely compromised LV dysfunction
- Mechanical complications of an AMI
- Complex coronary lesions (such as unprotected left main disease, complex multivessel disease, last remaining conduit vessel, and bypass graft disease)
- As a bridge to further therapy
- Prophylaxis in patients with severe left main coronary arterial stenosis
- Intractable myocardial ischemia
- Refractory heart failure
- Intractable ventricular arrhythmias
Intra-Aortic Balloon Pump (IABP)
- Based on principle of counterpulsation; balloon inflation during diastole increases central aortic pressure that can improve coronary blood flow, whereas deflation during systole decreases left ventricular afterload thus improving cardiac output.
- Can be inserted via 7.5 Fr sheath (30 cc, 40 cc) or 8 Fr Sheath (50 cc)
- Sizing is based on balloon volume and decided according to patient’s height (<5’4”: 30 or 40 cc)
- Fiber optic technology allows instantaneous signal transmission and automatic in-vivo calibration
Contraindications
- Severe aortic insufficiency
- Aortic aneurysm
- Aortic dissection
- Limb ischemia
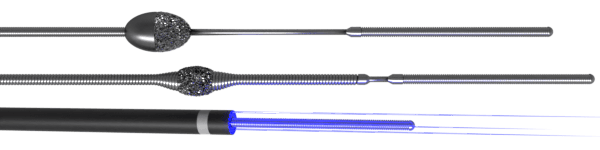
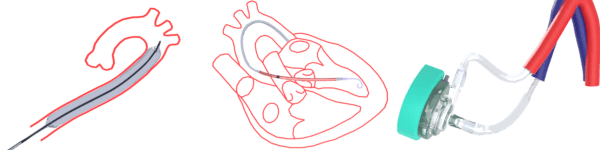
Impella Ventricular Assist Device (Abiomed Inc.)
With limited hemodynamics support from IABP, other percutaneous MCS devices are used in those required for better hemodynamic support.
- Microaxial pump directly draws blood from LV into aorta
- Provides direct LV unloading
- Impella 2.5 and CP inserted via 13 Fr and 14 Fr sheaths, respectively
- FDA indications:
- Impella 2.5, CP approved for temporary (<6 hours) ventricular support during high-risk PCI
- Impella 2.5, CP approved for short-term (<4 days) use and
- Impella 5.0, 5.5 and LD approved for <14 days use in cardiogenic shock following acute MI, open heart surgery, cardiomyopathy or myocarditis
- Impella RP: Inserted via 23 Fr venous access. Indicated for temporary (up to 14 days) RV support in patients with acute right heart failure or decompensation following LVAD implantation.
Contraindications
- Mural thrombus in the left ventricle
- Mechanical aortic valve or moderate to severe aortic stenosis (AVA of ≤1.5 cm 2 )
- Moderate to severe aortic insufficiency (echocardiographic grade ≥ 2+)
- Abdominal aneurysms
- Severe peripheral vascular disease or extreme tortuosity/calcifications
- Renal failure (creatinine ≥4 mg/dL)
- Liver dysfunction (platelet count ≤75,000/mm 3 or INR ≥2.0 or fibrinogen ≤1.50 g/L)
- Recent (<3 months) stroke or TIA
ECMO (Extracorporeal membrane oxygenation)
An extracorporeal membrane oxygenation (ECMO) device is a cardiopulmonary support system that consists of not only moving blood forward but also removes carbon dioxide as well as adds oxygen to venous blood using an artificial membrane lung.
- With venoarterial configuration (right-atrium-to-the-iliofemoral-artery system), the pulmonary circulation is bypassed and oxygenated blood returns to the patient via an arterial or venous route.
- With veno-venous bypass, ECMO is effective primarily as a therapeutic option for patients with severe respiratory failure.
- With venoarterial bypass, an extracorporeal pump is employed to support systemic perfusion, thus providing a hemodynamic support option in patients with circulatory and respiratory failure.
- VV ECMO (venous inflow, venous outflow) – used for acute respiratory failure
- VA ECMO (venous inflow, arterial outflow) – used for cardiogenic shock or cardiac arrest
- Venous cannulas are typically between 19-25 F and arterial cannulas are 15-24 F
Indications
- Acute hemodynamic deterioration such as cardiogenic shock and cardiopulmonary arrest with severe pulmonary congestion
- High-risk percutaneous coronary intervention
- Fulminant myocarditis presenting with cardiogenic shock
- Post-cardiotomy circulatory failure
- COVID-19 with severe respiratory failure
Contraindications
- Significant aortic regurgitation
- Severe peripheral artery disease
- Bleeding diathesis
- Recent cerebrovascular accident or head trauma
- Uncontrolled sepsis